当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rational Design of Chymotrypsin Inhibitor 2 by Optimizing Non-Native Interactions.
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2019-12-19 , DOI: 10.1021/acs.jcim.9b00911 Fernando B da Silva 1 , Vinícius M de Oliveira 2 , Murilo N Sanches 1 , Vinícius G Contessoto 3, 4 , Vitor B P Leite 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2019-12-19 , DOI: 10.1021/acs.jcim.9b00911 Fernando B da Silva 1 , Vinícius M de Oliveira 2 , Murilo N Sanches 1 , Vinícius G Contessoto 3, 4 , Vitor B P Leite 1
Affiliation
|
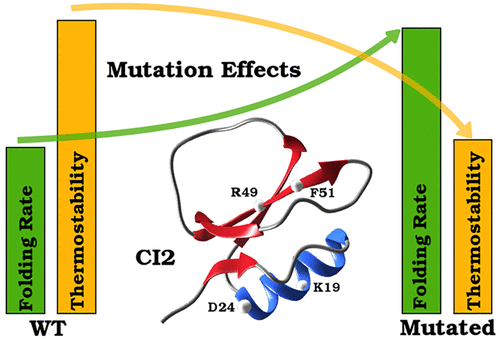
Rational design of proteins via mutagenesis is crucial for several biotechnological applications. A significant challenge of the computational strategies used to predict optimized mutations is to understand the influence of each amino acid during the folding process. In the present work, chymotrypsin inhibitor 2 (CI2) and several of its designed mutants have been simulated using a non-native hydrophobic and electrostatic potential as a structure-based Cα model. Through these simulations, we could identify the most critical folding stage to accelerate CI2 and also the charged residues responsible for providing its thermostability. The replacement of ionizable residues for hydrophobic ones tended to promote the formation of the CI2 secondary structure in the early transition state, which speeds up folding. However, this same replacement destabilized the native structure, and there was a decrease in the protein thermostability. Such a simple method proved to be capable of providing valuable information about thermodynamics and kinetics of CI2 and its mutations, thus being a fast alternative to the study of rational protein design.
中文翻译:

通过优化非天然相互作用,合理设计胰凝乳蛋白酶抑制剂2。
通过诱变合理设计蛋白质对几种生物技术应用至关重要。用于预测优化突变的计算策略的一项重大挑战是了解折叠过程中每种氨基酸的影响。在当前的工作中,使用非天然的疏水和静电势作为基于结构的Cα模型,对胰凝乳蛋白酶抑制剂2(CI2)及其几个设计的突变体进行了模拟。通过这些模拟,我们可以确定加速CI2的最关键的折叠阶段,并确定负责提供其热稳定性的带电残留物。用可电离的残基代替疏水残基倾向于在早期过渡状态下促进CI2二级结构的形成,从而加快折叠速度。然而,相同的替代物破坏了天然结构的稳定性,并且蛋白质的热稳定性降低了。事实证明,这种简单的方法能够提供有关CI2及其突变的热力学和动力学的有价值的信息,从而成为合理蛋白质设计研究的快速替代方法。
更新日期:2019-12-19
中文翻译:

通过优化非天然相互作用,合理设计胰凝乳蛋白酶抑制剂2。
通过诱变合理设计蛋白质对几种生物技术应用至关重要。用于预测优化突变的计算策略的一项重大挑战是了解折叠过程中每种氨基酸的影响。在当前的工作中,使用非天然的疏水和静电势作为基于结构的Cα模型,对胰凝乳蛋白酶抑制剂2(CI2)及其几个设计的突变体进行了模拟。通过这些模拟,我们可以确定加速CI2的最关键的折叠阶段,并确定负责提供其热稳定性的带电残留物。用可电离的残基代替疏水残基倾向于在早期过渡状态下促进CI2二级结构的形成,从而加快折叠速度。然而,相同的替代物破坏了天然结构的稳定性,并且蛋白质的热稳定性降低了。事实证明,这种简单的方法能够提供有关CI2及其突变的热力学和动力学的有价值的信息,从而成为合理蛋白质设计研究的快速替代方法。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号