当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermally Stable Half-Sandwich Benzhydryl Ln(II) (Ln = Sm, Yb) Complexes Supported by Sterically Demanding Carbazolyl and Fluorenyl Ligands
Organometallics ( IF 2.5 ) Pub Date : 2019-12-03 , DOI: 10.1021/acs.organomet.9b00624 Alexander N. Selikhov 1, 2 , Andrey S. Shavyrin 1 , Anton V. Cherkasov 1 , Georgy K. Fukin 1 , Alexander A. Trifonov 1, 2
Organometallics ( IF 2.5 ) Pub Date : 2019-12-03 , DOI: 10.1021/acs.organomet.9b00624 Alexander N. Selikhov 1, 2 , Andrey S. Shavyrin 1 , Anton V. Cherkasov 1 , Georgy K. Fukin 1 , Alexander A. Trifonov 1, 2
Affiliation
|
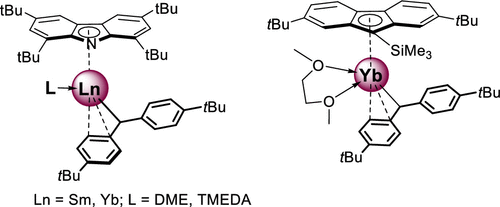
A series of new isolable and thermally stable half-sandwich Ln(II) benzhydryl complexes coordinated by the sterically demanding ligands tert-butylcarbazol-9-yl [tBu4Carb]Ln[(p-tBu-C6H4)2CH](L) (Ln = Sm, L = DME (4); Ln = Yb, L = DME (5); Ln = Yb, L = TMEDA (6)) and 2,7-di-tert-butyl-fluoren-9-trimethylsilylyl [2,7-tBu2-9-Me3Si-C13H6]Yb[(p-tBu-C6H4)2CH](DME) (7) were synthesized by the alkane elimination reaction of [(p-tBu-C6H4)2CH]2Ln(Ln) (Ln = Sm, Yb) with tBu4CarbH and 2,7-tBu2-9-Me3Si-C13H7. X-ray analysis revealed that in 4, 5, and 7 the benzhydryl ligand is coordinated to the metal ion in an η3 coordination mode, while in 6 it is η1-bound. The type of coordination of the benzhydryl ligands in diamagnetic 5–7 is retained in their C6D6 solutions. Complexes 4–7 demonstrated unprecedented thermal stability and do not undergo decomposition after heating their solutions in C6D6 or toluene at 100 °C for 72 h. The reactions of [tBu4Carb]Ln[(p-tBu-C6H4)2CH](DME) (Ln = Sm (4), Ln = Yb (5)) with an excess of DME led to the formation of the symmetrical bis(carbazolyl) complex products [tBu4Carb]2Ln(DME)4 (Ln = Sm (8), Yb (9)) isolated in the form of separated ion pairs.
中文翻译:

热稳定的半三明治式苯甲酰基Ln(II)(Ln = Sm,Yb)配合物,由立体要求的咔唑基和芴基配体支持
一系列新的可分离且热稳定的半三明治Ln(II)苯二甲酰配合物,由立体要求高的配体叔丁基咔唑-9-基[ t Bu 4 Carb] Ln [(p - t Bu-C 6 H 4)2配位CH](L)(Ln = Sm,L = DME(4); Ln = Yb,L = DME(5); Ln = Yb,L = TMEDA(6))和2,7-二叔丁基-芴-9-基trimethylsilylyl [2,7-吨卜2 -9-ME 3的Si-C 13 H ^ 6 ]镱[(p -吨卜-C通过[(pt Bu-C 6 H 4)2 CH] 2 Ln(L n)(Ln = Sm,Yb)与t Bu的烷烃消除反应合成6 H 4)2 CH](DME)(7)4 CarbH和2,7 - t Bu 2 -9-Me 3 Si-C 13 H 7。X射线分析表明,在4,5,和7的二苯甲基配位体配位到在η与金属离子3配位模式,而在6它是η 1分结合的。在抗磁二苯甲基配位体的配位类型5 - 7被保持在它们的C 6 d 6解决方案。配合物4 – 7具有前所未有的热稳定性,并且将其在C 6 D 6或甲苯中的溶液在100°C下加热72小时后不会分解。[ t Bu 4 Carb] Ln [(p - t Bu-C 6 H 4)2 CH](DME)(Ln = Sm(4),Ln = Yb(5))与过量的DME导致形成对称的双(咔唑基)复杂产物[ t Bu 4 Carb] 2 Ln(DME)4(Ln = Sm(8),Yb(9)),形式为分离的离子对。
更新日期:2019-12-04
中文翻译:

热稳定的半三明治式苯甲酰基Ln(II)(Ln = Sm,Yb)配合物,由立体要求的咔唑基和芴基配体支持
一系列新的可分离且热稳定的半三明治Ln(II)苯二甲酰配合物,由立体要求高的配体叔丁基咔唑-9-基[ t Bu 4 Carb] Ln [(p - t Bu-C 6 H 4)2配位CH](L)(Ln = Sm,L = DME(4); Ln = Yb,L = DME(5); Ln = Yb,L = TMEDA(6))和2,7-二叔丁基-芴-9-基trimethylsilylyl [2,7-吨卜2 -9-ME 3的Si-C 13 H ^ 6 ]镱[(p -吨卜-C通过[(pt Bu-C 6 H 4)2 CH] 2 Ln(L n)(Ln = Sm,Yb)与t Bu的烷烃消除反应合成6 H 4)2 CH](DME)(7)4 CarbH和2,7 - t Bu 2 -9-Me 3 Si-C 13 H 7。X射线分析表明,在4,5,和7的二苯甲基配位体配位到在η与金属离子3配位模式,而在6它是η 1分结合的。在抗磁二苯甲基配位体的配位类型5 - 7被保持在它们的C 6 d 6解决方案。配合物4 – 7具有前所未有的热稳定性,并且将其在C 6 D 6或甲苯中的溶液在100°C下加热72小时后不会分解。[ t Bu 4 Carb] Ln [(p - t Bu-C 6 H 4)2 CH](DME)(Ln = Sm(4),Ln = Yb(5))与过量的DME导致形成对称的双(咔唑基)复杂产物[ t Bu 4 Carb] 2 Ln(DME)4(Ln = Sm(8),Yb(9)),形式为分离的离子对。

































 京公网安备 11010802027423号
京公网安备 11010802027423号