当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Filling the void: controlled donor-acceptor interaction facilitates the formation of an M-M single bond in the zero oxidation state of M (M = Zn, Cd, Hg).
Dalton Transactions ( IF 3.5 ) Pub Date : 2019-12-18 , DOI: 10.1039/c9dt04213j Ranajit Saha 1 , Sudip Pan , Pratim K Chattaraj , Gabriel Merino
Dalton Transactions ( IF 3.5 ) Pub Date : 2019-12-18 , DOI: 10.1039/c9dt04213j Ranajit Saha 1 , Sudip Pan , Pratim K Chattaraj , Gabriel Merino
Affiliation
|
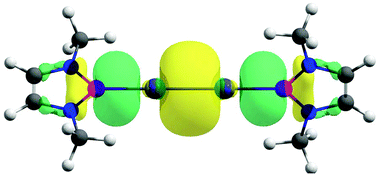
The intriguing question of whether it is possible to form a genuine M0-M0 single bond for the M2 species (M = Zn, Cd, Hg) is addressed here. So far, all the bonds reported in the literature are exclusively MI-MI. Herein, we present viable M2(NHBMe)2 (M = Zn, Cd, Hg; NHBMe = (HCNMe)2B) complexes in which the controlled donor-acceptor interaction leads to an M0-M0 single bond. In these complexes, M2 in the 1∑g ground state with the (nσg+)2(nσu+)2 (n = 7, 10 and 14 for M = Zn, Cd and Hg, respectively) valence electron configuration forms donor-acceptor bonding with singlet 2NHBMe ligands where a combined effect of dominant (+,-) σ-backdonation from the antibonding (nσu+)2 orbital of M2 to the 2NHBMe ligands and a somewhat weaker (+,+) σ-donation from the 2NHBMe ligands to the bonding (n + 1)σg+ orbital leads to the unorthodox bonding situation of forming an M-M single bond in the zero oxidation state by eventually nullifying one effect by another. This is an unprecedented situation in the sense that the NHBMe ligand acts as a strong σ-acceptor and a weaker σ-donor. A comparison with the experimentally reported M2(PhDipp)2 complexes reveals the uniqueness of the NHBMe ligand in exhibiting such a bonding scenario. The M2(NHBMe)2 complex is thermochemically viable with respect to possible dissociation channels at room temperature, except for metal extrusion processes, M2(NHBMe)2 → M + M(NHBMe)2 and M2(NHBMe)2 → M2 + (NHBMe)2. Although the latter two processes are exergonic, they are kinetically protected by a high free energy barrier of 26.5-39.5 kcal mol-1. The experimental characterization of M2(PhDipp)2 despite similar exergonic channels reveals such kinetic stability to be enough for the viability of the M2(NHBMe)2 complexes. Furthermore, the ligand exchange reaction considering M2(PhMe)2 as the starting material also turned out to be feasible. Therefore, the M2(NHBMe)2 complexes are the first cases that feature a neutral M2 moiety with a single M0-M0 covalent bond, where M is a Group 12 metal.
中文翻译:

填充空隙:受控的供体-受体相互作用促进了M的零氧化态(M = Zn,Cd,Hg)中MM单键的形成。
这里解决了一个有趣的问题,即是否有可能为M2物种(M = Zn,Cd,Hg)形成真正的M0-M0单键。到目前为止,文献中报道的所有键都是MI-MI。在这里,我们提出了可行的M2(NHBMe)2(M = Zn,Cd,Hg; NHBMe =(HCNMe)2B)复合物,其中受控的供体-受体相互作用导致M0-M0单键。在这些络合物中,基态为1∑g的M2与(nσg+)2(nσu+)2(M = Zn,Cd和Hg分别为n = 7、10和14)价电子构型形成施主-受主键单线态2NHBMe配体,其中M2的反键(nσu+)2轨道与2NHBMe配体的显性(+,-)σ背向结合作用和较弱的(+,+)从2NHBMe配体到键(n + 1)σg+轨道的σ捐赠导致了一种非正统的键合情况,即最终通过使一个效应彼此抵消而在零氧化状态下形成MM单键。从NHBMe配体充当强σ受体和弱σ受体的意义上来说,这是前所未有的情况。与实验报告的M2(PhDipp)2配合物进行比较,发现NHBMe配体在表现出这种键合情况时具有独特性。M2(NHBMe)2配合物在室温下相对于可能的解离通道在热化学上可行,但金属挤压工艺除外,M2(NHBMe)2→M + M(NHBMe)2和M2(NHBMe)2→M2 +(NHBMe )2。尽管后两个过程是能动的,但它们在动力学上受到26.5-39.5 kcal mol-1的高自由能垒的保护。尽管有相似的运动通道,但M2(PhDipp)2的实验表征表明,这种动力学稳定性足以满足M2(NHBMe)2配合物的活力。此外,以M 2(PhMe)2为原料的配体交换反应也被证明是可行的。因此,M2(NHBMe)2配合物是第一种具有中性M2部分和单个M0-M0共价键的复合物,其中M为第12组金属。
更新日期:2019-12-19
中文翻译:

填充空隙:受控的供体-受体相互作用促进了M的零氧化态(M = Zn,Cd,Hg)中MM单键的形成。
这里解决了一个有趣的问题,即是否有可能为M2物种(M = Zn,Cd,Hg)形成真正的M0-M0单键。到目前为止,文献中报道的所有键都是MI-MI。在这里,我们提出了可行的M2(NHBMe)2(M = Zn,Cd,Hg; NHBMe =(HCNMe)2B)复合物,其中受控的供体-受体相互作用导致M0-M0单键。在这些络合物中,基态为1∑g的M2与(nσg+)2(nσu+)2(M = Zn,Cd和Hg分别为n = 7、10和14)价电子构型形成施主-受主键单线态2NHBMe配体,其中M2的反键(nσu+)2轨道与2NHBMe配体的显性(+,-)σ背向结合作用和较弱的(+,+)从2NHBMe配体到键(n + 1)σg+轨道的σ捐赠导致了一种非正统的键合情况,即最终通过使一个效应彼此抵消而在零氧化状态下形成MM单键。从NHBMe配体充当强σ受体和弱σ受体的意义上来说,这是前所未有的情况。与实验报告的M2(PhDipp)2配合物进行比较,发现NHBMe配体在表现出这种键合情况时具有独特性。M2(NHBMe)2配合物在室温下相对于可能的解离通道在热化学上可行,但金属挤压工艺除外,M2(NHBMe)2→M + M(NHBMe)2和M2(NHBMe)2→M2 +(NHBMe )2。尽管后两个过程是能动的,但它们在动力学上受到26.5-39.5 kcal mol-1的高自由能垒的保护。尽管有相似的运动通道,但M2(PhDipp)2的实验表征表明,这种动力学稳定性足以满足M2(NHBMe)2配合物的活力。此外,以M 2(PhMe)2为原料的配体交换反应也被证明是可行的。因此,M2(NHBMe)2配合物是第一种具有中性M2部分和单个M0-M0共价键的复合物,其中M为第12组金属。











































 京公网安备 11010802027423号
京公网安备 11010802027423号