当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Privilege-Structure-Oriented Three-Component Asymmetric Aminomethylation: Assembly of Chiral 3-Aminomethyl Indolones.
Organic Letters ( IF 4.9 ) Pub Date : 2019-12-03 , DOI: 10.1021/acs.orglett.9b03787
Zhenghui Kang 1 , Dan Zhang 1 , Xinfang Xu 1 , Wenhao Hu 1
Organic Letters ( IF 4.9 ) Pub Date : 2019-12-03 , DOI: 10.1021/acs.orglett.9b03787
Zhenghui Kang 1 , Dan Zhang 1 , Xinfang Xu 1 , Wenhao Hu 1
Affiliation
|
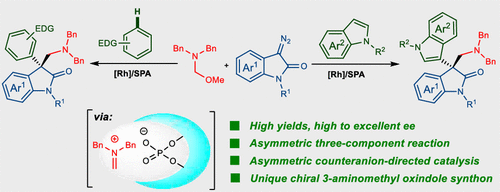
The asymmetric aminomethylation reaction of 3-diazooxindoles with electronic-rich arenes and N,O-acetals cooperatively catalyzed by achiral dirhodium complex and chiral phosphoric acid is reported. The reaction provides a novel method for the facile synthesis of chiral 3-aminomethyl oxindoles with an all-carbon quaternary center in good yields (82-98%) with high to excellent enantioselectivities (up to 97% ee). The transformation proceeds through a convergent addition of a reactive zwitterionic intermediate with a chiral methylene iminium generated in situ via asymmetric counteranion-directed catalysis (ACDC). This work represents the first asymmetric aminomethylation method of mixed 3,3'-bisindoles with structural diversity.
中文翻译:

面向特权结构的三组分不对称氨甲基化:手性3-氨甲基吲哚酮的组装。
报道了非手性吡啶鎓配合物和手性磷酸协同催化3-重氮杂吲哚与富电子芳烃和N,O-乙缩醛的不对称氨甲基化反应。该反应提供了一种易于合成具有全碳季中心的手性3-氨基甲基羟吲哚的新方法,该方法的收率高(82-98%),对映选择性高(至97%ee)。该转化通过向反应性两性离子中间体与通过不对称抗衡阴离子定向催化(ACDC)原位产生的手性亚甲基亚胺的会聚加成而进行。这项工作代表了具有结构多样性的混合3,3'-双吲哚的第一种不对称氨基甲基化方法。
更新日期:2019-12-04
中文翻译:

面向特权结构的三组分不对称氨甲基化:手性3-氨甲基吲哚酮的组装。
报道了非手性吡啶鎓配合物和手性磷酸协同催化3-重氮杂吲哚与富电子芳烃和N,O-乙缩醛的不对称氨甲基化反应。该反应提供了一种易于合成具有全碳季中心的手性3-氨基甲基羟吲哚的新方法,该方法的收率高(82-98%),对映选择性高(至97%ee)。该转化通过向反应性两性离子中间体与通过不对称抗衡阴离子定向催化(ACDC)原位产生的手性亚甲基亚胺的会聚加成而进行。这项工作代表了具有结构多样性的混合3,3'-双吲哚的第一种不对称氨基甲基化方法。

































 京公网安备 11010802027423号
京公网安备 11010802027423号