Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Proliferating Transitory T Cells with an Effector-like Transcriptional Signature Emerge from PD-1+ Stem-like CD8+ T Cells during Chronic Infection.
Immunity ( IF 25.5 ) Pub Date : 2019-12-03 , DOI: 10.1016/j.immuni.2019.11.002 William H Hudson 1 , Julia Gensheimer 1 , Masao Hashimoto 1 , Andreas Wieland 1 , Rajesh M Valanparambil 1 , Peng Li 2 , Jian-Xin Lin 2 , Bogumila T Konieczny 1 , Se Jin Im 1 , Gordon J Freeman 3 , Warren J Leonard 2 , Haydn T Kissick 4 , Rafi Ahmed 1
Immunity ( IF 25.5 ) Pub Date : 2019-12-03 , DOI: 10.1016/j.immuni.2019.11.002 William H Hudson 1 , Julia Gensheimer 1 , Masao Hashimoto 1 , Andreas Wieland 1 , Rajesh M Valanparambil 1 , Peng Li 2 , Jian-Xin Lin 2 , Bogumila T Konieczny 1 , Se Jin Im 1 , Gordon J Freeman 3 , Warren J Leonard 2 , Haydn T Kissick 4 , Rafi Ahmed 1
Affiliation
|
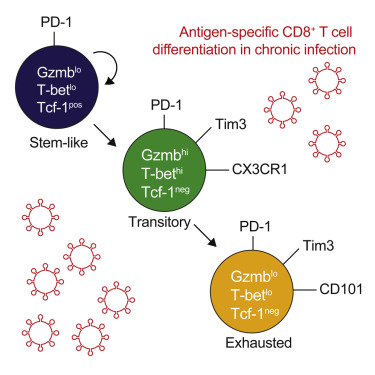
T cell dysfunction is a characteristic feature of chronic viral infection and cancer. Recent studies in chronic lymphocytic choriomeningitis virus (LCMV) infection have defined a PD-1+ Tcf-1+ CD8+ T cell subset capable of self-renewal and differentiation into more terminally differentiated cells that downregulate Tcf-1 and express additional inhibitory molecules such as Tim3. Here, we demonstrated that expression of the glycoprotein CD101 divides this terminally differentiated population into two subsets. Stem-like Tcf-1+ CD8+ T cells initially differentiated into a transitory population of CD101-Tim3+ cells that later converted into CD101+ Tim3+ cells. Recently generated CD101-Tim3+ cells proliferated in vivo, contributed to viral control, and were marked by an effector-like transcriptional signature including expression of the chemokine receptor CX3CR1, pro-inflammatory cytokines, and granzyme B. PD-1 pathway blockade increased the numbers of CD101-Tim3+ CD8+ T cells, suggesting that these newly generated transitional cells play a critical role in PD-1-based immunotherapy.
中文翻译:

慢性感染期间,PD-1+ 干细胞样 CD8+ T 细胞产生具有效应样转录特征的增殖性瞬时 T 细胞。
T 细胞功能障碍是慢性病毒感染和癌症的一个特征。最近对慢性淋巴细胞脉络丛脑膜炎病毒 (LCMV) 感染的研究已经定义了 PD-1+ Tcf-1+ CD8+ T 细胞亚群,能够自我更新并分化为更多终末分化细胞,下调 Tcf-1 并表达其他抑制分子,例如蒂姆3.在这里,我们证明了糖蛋白 CD101 的表达将这个终末分化群体分为两个子集。干细胞样 Tcf-1+ CD8+ T 细胞最初分化为短暂的 CD101-Tim3+ 细胞群,随后转化为 CD101+ Tim3+ 细胞。最近生成的 CD101-Tim3+ 细胞在体内增殖,有助于病毒控制,并具有类似效应子的转录特征,包括趋化因子受体 CX3CR1、促炎细胞因子和颗粒酶 B 的表达。PD-1 通路阻断增加了细胞数量CD101-Tim3+ CD8+ T 细胞,表明这些新产生的过渡细胞在基于 PD-1 的免疫疗法中发挥着关键作用。
更新日期:2019-12-04
中文翻译:

慢性感染期间,PD-1+ 干细胞样 CD8+ T 细胞产生具有效应样转录特征的增殖性瞬时 T 细胞。
T 细胞功能障碍是慢性病毒感染和癌症的一个特征。最近对慢性淋巴细胞脉络丛脑膜炎病毒 (LCMV) 感染的研究已经定义了 PD-1+ Tcf-1+ CD8+ T 细胞亚群,能够自我更新并分化为更多终末分化细胞,下调 Tcf-1 并表达其他抑制分子,例如蒂姆3.在这里,我们证明了糖蛋白 CD101 的表达将这个终末分化群体分为两个子集。干细胞样 Tcf-1+ CD8+ T 细胞最初分化为短暂的 CD101-Tim3+ 细胞群,随后转化为 CD101+ Tim3+ 细胞。最近生成的 CD101-Tim3+ 细胞在体内增殖,有助于病毒控制,并具有类似效应子的转录特征,包括趋化因子受体 CX3CR1、促炎细胞因子和颗粒酶 B 的表达。PD-1 通路阻断增加了细胞数量CD101-Tim3+ CD8+ T 细胞,表明这些新产生的过渡细胞在基于 PD-1 的免疫疗法中发挥着关键作用。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号