当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Insight toward Understanding the Role of Charge in Thiourea Organocatalysis.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-12-16 , DOI: 10.1021/acs.joc.9b02682 Ivor Smajlagic 1 , Matt Guest 1 , Rocío Durán 2 , Barbara Herrera 2 , Travis Dudding 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-12-16 , DOI: 10.1021/acs.joc.9b02682 Ivor Smajlagic 1 , Matt Guest 1 , Rocío Durán 2 , Barbara Herrera 2 , Travis Dudding 1
Affiliation
|
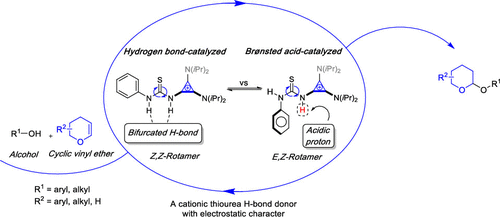
Pyranylation and glycosylation are pivotal for accessing a myriad of natural products, pharmaceuticals, and drug candidates. Catalytic approaches for enabling these transformations are of utmost importance and integral to advancing this area of synthesis. In exploring this chemical space, a combined experimental and computational mechanistic study of pyranylation and 2-deoxygalactosylation catalyzed by a cationic thiourea organocatalyst is reported. To this end, a thiourea-cyclopropenium organocatalyst was employed as a model system in combination with an arsenal of mechanistic techniques, including 13C kinetic isotope effect experiments, deuterated labeling studies, variable-temperature 1H NMR spectroscopy, and density functional theory calculations. From these studies, two distinct reaction pathways were identified for this transformation corresponding to either dual hydrogen bond (H-bond) activation or Brønsted acid catalysis. The former involving thiourea orchestrated bifurcated hydrogen bonding proceeded in an asynchronous concerted fashion. In contrast, the latter stepwise mechanism involving Brønsted acid catalysis hinged upon the formation of an oxocarbenium intermediate accompanied by subsequent alcohol addition.
中文翻译:

机械理解,以了解电荷在硫脲有机催化中的作用。
吡喃基化和糖基化对于获取多种天然产物,药物和候选药物至关重要。促成这些转化的催化方法对于推进这一合成领域至关重要,并且是不可或缺的。在探索该化学空间中,报道了由阳离子硫脲有机催化剂催化的吡喃基化和2-脱氧半乳糖基化的组合实验和计算机理的研究。为此,将硫脲-环丙烯有机催化剂与一系列机械技术结合起来用作模型系统,包括13C动力学同位素效应实验,氘代标记研究,可变温度1H NMR光谱和密度泛函理论计算。根据这些研究,对于该转化,确定了两个不同的反应途径,分别对应于双氢键(H键)活化或布朗斯台德酸催化。前者涉及硫脲精心设计的分叉氢键,以异步协同方式进行。相反,后者涉及布朗斯台德酸催化的逐步机理取决于氧羰基中间体的形成以及随后的醇加成。
更新日期:2019-12-17
中文翻译:

机械理解,以了解电荷在硫脲有机催化中的作用。
吡喃基化和糖基化对于获取多种天然产物,药物和候选药物至关重要。促成这些转化的催化方法对于推进这一合成领域至关重要,并且是不可或缺的。在探索该化学空间中,报道了由阳离子硫脲有机催化剂催化的吡喃基化和2-脱氧半乳糖基化的组合实验和计算机理的研究。为此,将硫脲-环丙烯有机催化剂与一系列机械技术结合起来用作模型系统,包括13C动力学同位素效应实验,氘代标记研究,可变温度1H NMR光谱和密度泛函理论计算。根据这些研究,对于该转化,确定了两个不同的反应途径,分别对应于双氢键(H键)活化或布朗斯台德酸催化。前者涉及硫脲精心设计的分叉氢键,以异步协同方式进行。相反,后者涉及布朗斯台德酸催化的逐步机理取决于氧羰基中间体的形成以及随后的醇加成。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号