当前位置:
X-MOL 学术
›
Cell Death Differ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Competitive ubiquitination activates the tumor suppressor p53.
Cell Death and Differentiation ( IF 13.7 ) Pub Date : 2019-12-02 , DOI: 10.1038/s41418-019-0463-x
Xingyao Li 1 , Mengqi Guo 2 , Lun Cai 1 , Tingting Du 1, 3 , Ying Liu 3 , Han-Fei Ding 1, 4 , Hongbo Wang 2 , Junran Zhang 5 , Xiaoguang Chen 3 , Chunhong Yan 1, 6
Cell Death and Differentiation ( IF 13.7 ) Pub Date : 2019-12-02 , DOI: 10.1038/s41418-019-0463-x
Xingyao Li 1 , Mengqi Guo 2 , Lun Cai 1 , Tingting Du 1, 3 , Ying Liu 3 , Han-Fei Ding 1, 4 , Hongbo Wang 2 , Junran Zhang 5 , Xiaoguang Chen 3 , Chunhong Yan 1, 6
Affiliation
|
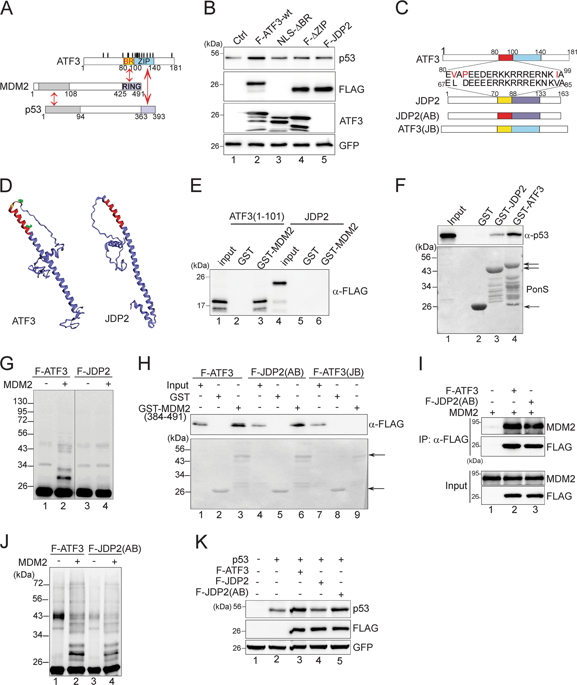
Blocking p53 ubiquitination through disrupting its interaction with MDM2 or inhibiting the MDM2 catalytic activity is the central mechanism by which the tumor suppressor p53 is activated in response to genotoxic challenges. Although MDM2 is first characterized as the major E3 ubiquitin ligase for p53, it can also catalyze the conjugation of ubiquitin moieties to other proteins (e.g., activating transcription factor 3, or ATF3). Here we report that ATF3 can act as an ubiquitin "trap" and competes with p53 for MDM2-mediated ubiquitination. While ATF3-mediated p53 stabilization required ATF3 binding to the MDM2 RING domain, we demonstrated that ATF3 ubiquitination catalyzed by MDM2 was indispensable for p53 activation in response to DNA damage. Moreover, a cancer-derived ATF3 mutant (R88G) devoid of ubiquitination failed to prevent p53 from MDM2-mediated degradation and thus was unable to activate the tumor suppressor. Therefore, we have identified a previously-unknown mechanism that can activate p53 in the genotoxic response.
中文翻译:

竞争性泛素化激活肿瘤抑制基因 p53。
通过破坏 p53 与 MDM2 的相互作用或抑制 MDM2 催化活性来阻断 p53 泛素化是肿瘤抑制基因 p53 响应遗传毒性挑战而被激活的核心机制。虽然 MDM2 最初被表征为 p53 的主要 E3 泛素连接酶,但它也可以催化泛素部分与其他蛋白质(例如,激活转录因子 3 或 ATF3)的结合。在这里,我们报道了 ATF3 可以充当泛素“陷阱”,并与 p53 竞争 MDM2 介导的泛素化。虽然 ATF3 介导的 p53 稳定需要 ATF3 与 MDM2 RING 结构域结合,但我们证明 MDM2 催化的 ATF3 泛素化对于响应 DNA 损伤的 p53 激活是必不可少的。此外,缺乏泛素化的癌症来源的 ATF3 突变体 (R88G) 未能阻止 p53 从 MDM2 介导的降解中消失,因此无法激活肿瘤抑制因子。因此,我们确定了一种以前未知的机制,可以在遗传毒性反应中激活 p53。
更新日期:2019-12-03
中文翻译:

竞争性泛素化激活肿瘤抑制基因 p53。
通过破坏 p53 与 MDM2 的相互作用或抑制 MDM2 催化活性来阻断 p53 泛素化是肿瘤抑制基因 p53 响应遗传毒性挑战而被激活的核心机制。虽然 MDM2 最初被表征为 p53 的主要 E3 泛素连接酶,但它也可以催化泛素部分与其他蛋白质(例如,激活转录因子 3 或 ATF3)的结合。在这里,我们报道了 ATF3 可以充当泛素“陷阱”,并与 p53 竞争 MDM2 介导的泛素化。虽然 ATF3 介导的 p53 稳定需要 ATF3 与 MDM2 RING 结构域结合,但我们证明 MDM2 催化的 ATF3 泛素化对于响应 DNA 损伤的 p53 激活是必不可少的。此外,缺乏泛素化的癌症来源的 ATF3 突变体 (R88G) 未能阻止 p53 从 MDM2 介导的降解中消失,因此无法激活肿瘤抑制因子。因此,我们确定了一种以前未知的机制,可以在遗传毒性反应中激活 p53。

































 京公网安备 11010802027423号
京公网安备 11010802027423号