Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, and Anticancer Activity of Novel Trimethoxyphenyl-Derived Chalcone-Benzimidazolium Salts.
ACS Omega ( IF 3.7 ) Pub Date : 2019-11-20 , DOI: 10.1021/acsomega.9b03077 Jun-Li Yang 1, 2 , Yong-Hui Ma 1 , Yan-Hua Li 2 , Yi-Peng Zhang 1 , Hong-Chang Tian 1 , Yuan-Cheng Huang 1 , Yan Li 3 , Wen Chen 1 , Li-Juan Yang 2
ACS Omega ( IF 3.7 ) Pub Date : 2019-11-20 , DOI: 10.1021/acsomega.9b03077 Jun-Li Yang 1, 2 , Yong-Hui Ma 1 , Yan-Hua Li 2 , Yi-Peng Zhang 1 , Hong-Chang Tian 1 , Yuan-Cheng Huang 1 , Yan Li 3 , Wen Chen 1 , Li-Juan Yang 2
Affiliation
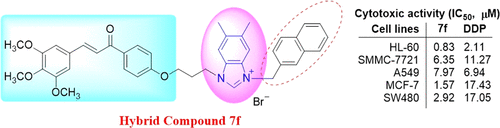
|
A series of novel trimethoxyphenyl-derived chalcone-benzimidazolium salts were synthesized. The biological properties of the compounds were screened in vitro against five different human tumor cell lines. The results suggest that the 5,6-dimethyl-benzimidazole or 2-methyl-benzimidazole ring as well as the 2-naphthylmethyl, 4-methylbenzyl, or 2-naphthylacyl substituent at position-3 of the benzimidazole ring was important to the cytotoxic activity. Notably, (E)-5,6-dimethyl-3-(naphthalen-2-ylmethyl)-1-(3-(4-(3-(3,4,5-trimethoxyphenyl)acryloyl)phenoxy)propyl)-1H-benzo[d]imidazol-3-ium bromide (7f) was more selective to HL-60, MCF-7, and SW-480 cell lines with IC50 values 8.0-, 11.1-, and 5.8-fold lower than DDP. Studies of the antitumor mechanism of action showed that compound 7f could induce cell-cycle G1 phase arrest and apoptosis in SMMC-7721 cells.
中文翻译:

新型三甲氧基苯基衍生的查尔酮-苯并咪唑鎓盐的设计,合成和抗癌活性。
合成了一系列新颖的三甲氧基苯基衍生的查尔酮-苯并咪唑鎓盐。在体外针对五种不同的人类肿瘤细胞系筛选了该化合物的生物学特性。结果表明5,6-二甲基苯并咪唑环或2-甲基苯并咪唑环以及苯并咪唑环的3位上的2-萘甲基,4-甲基苄基或2-萘甲酰基取代基对细胞毒活性至关重要。值得注意的是,(E)-5,6-二甲基-3-(萘-2-基甲基)-1-(3-(4-(3-(3,4,5-三甲氧基苯基)丙烯酰基)苯氧基)丙基)-1H -苯并[d]咪唑-3-溴化铵(7f)对HL-60,MCF-7和SW-480细胞系的选择性更高,IC50值分别比DDP低8.0-,11.1和5.8倍。
更新日期:2019-12-03
中文翻译:

新型三甲氧基苯基衍生的查尔酮-苯并咪唑鎓盐的设计,合成和抗癌活性。
合成了一系列新颖的三甲氧基苯基衍生的查尔酮-苯并咪唑鎓盐。在体外针对五种不同的人类肿瘤细胞系筛选了该化合物的生物学特性。结果表明5,6-二甲基苯并咪唑环或2-甲基苯并咪唑环以及苯并咪唑环的3位上的2-萘甲基,4-甲基苄基或2-萘甲酰基取代基对细胞毒活性至关重要。值得注意的是,(E)-5,6-二甲基-3-(萘-2-基甲基)-1-(3-(4-(3-(3,4,5-三甲氧基苯基)丙烯酰基)苯氧基)丙基)-1H -苯并[d]咪唑-3-溴化铵(7f)对HL-60,MCF-7和SW-480细胞系的选择性更高,IC50值分别比DDP低8.0-,11.1和5.8倍。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号