当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility Behavior of Calcium Sulfate Dihydrate (Gypsum) in an Aqueous Sodium Chloride System in the Presence of Hydroxyalkyl Ammonium Acetate Ionic Liquids Additives: Morphology Changes and Physicochemical Solution Properties at 35 °C
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2019-12-03 , DOI: 10.1021/acs.jced.9b00354 Jignesh Shukla 1 , Mohit J. Mehta 1 , Arvind Kumar 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2019-12-03 , DOI: 10.1021/acs.jced.9b00354 Jignesh Shukla 1 , Mohit J. Mehta 1 , Arvind Kumar 1
Affiliation
|
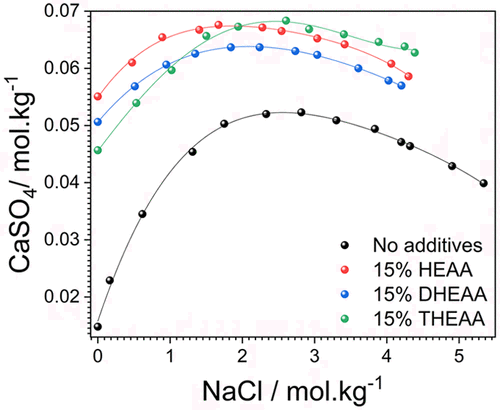
Dissolution characteristics of calcium sulfate dihydrate (gypsum, CaSO4·2H2O) in brines have been investigated in the presence of hydroxyalkyl ammonium acetate ionic liquids (ILs): hydroxyethylammonium acetate (HEAA), dihydroxyethylammonium acetate (DHEAA), or trihydroxyethylammonium acetate (THEAA) at 35 °C. The addition of ILs increased solubility significantly following the order: HEAA > DHEAA > THEAA. Solubility maximum of CaSO4·2H2O shifted toward a lower concentration of NaCl in solution with the addition of ILs. Mean ionic activity coefficients γ± of CaSO4·2H2O have been estimated as a function of solution ionic strength using the extended Debye–Hückel law with quadratic terms. The solubility product was calculated using a solubility equilibrium of CaSO4·2H2O. Density (ρ) and speed of sound (u) of (CaSO4·2H2O + NaCl + ILs + H2O) system have been recorded at 35 °C. Primary data on u and ρ were used to derive solution isentropic compressibility (κS). Polynomial equations have been used to fit the measured or derived properties. Dissolution kinetics of gypsum in aqueous ILs solution have been measured using in situ IR analysis, and the morphology of gypsum crystals obtained from various conditions have been examined by recording SEM images. SEM images show both classical and nonclassical crystallization behavior of gypsum, depending on the nature of the IL.
中文翻译:

羟烷基乙酸铵铵离子液体添加剂存在下二水合硫酸钙在氯化钠水溶液中的溶解行为:35°C时的形态变化和理化溶液性质
在乙酸羟烷基铵离子液体(ILs)的存在下,研究了二水合硫酸钙(石膏,CaSO 4 ·2H 2 O)在盐水中的溶解特性:乙酸羟乙基铵(HEAA),乙酸二羟乙基铵(DHEAA)或三羟乙基乙酸铵( THEAA)在35°C下。IL的添加按照以下顺序显着增加了溶解度:HEAA> DHEAA> THEAA。添加ILs后,CaSO 4 ·2H 2 O的最大溶解度向溶液中的NaCl浓度降低。CaSO 4 ·2H 2的平均离子活度系数γ ±使用扩展的Debye-Hückel定律和二次项,可以将O估计为溶液离子强度的函数。使用CaSO 4 ·2H 2 O的溶解度平衡来计算溶解度乘积。(CaSO 4 ·2H 2 O + NaCl + ILs + H 2 O)系统的密度(ρ)和声速(u)已记录在35°C。初级数据ù和ρ被用来推导溶液等熵压缩(κ小号)。多项式方程式已用于拟合测量或推导的属性。使用原位红外分析测量了石膏在ILs水溶液中的溶解动力学,并通过记录SEM图像检查了从各种条件获得的石膏晶体的形态。SEM图像显示了石膏的经典和非经典结晶行为,这取决于IL的性质。
更新日期:2019-12-03
中文翻译:

羟烷基乙酸铵铵离子液体添加剂存在下二水合硫酸钙在氯化钠水溶液中的溶解行为:35°C时的形态变化和理化溶液性质
在乙酸羟烷基铵离子液体(ILs)的存在下,研究了二水合硫酸钙(石膏,CaSO 4 ·2H 2 O)在盐水中的溶解特性:乙酸羟乙基铵(HEAA),乙酸二羟乙基铵(DHEAA)或三羟乙基乙酸铵( THEAA)在35°C下。IL的添加按照以下顺序显着增加了溶解度:HEAA> DHEAA> THEAA。添加ILs后,CaSO 4 ·2H 2 O的最大溶解度向溶液中的NaCl浓度降低。CaSO 4 ·2H 2的平均离子活度系数γ ±使用扩展的Debye-Hückel定律和二次项,可以将O估计为溶液离子强度的函数。使用CaSO 4 ·2H 2 O的溶解度平衡来计算溶解度乘积。(CaSO 4 ·2H 2 O + NaCl + ILs + H 2 O)系统的密度(ρ)和声速(u)已记录在35°C。初级数据ù和ρ被用来推导溶液等熵压缩(κ小号)。多项式方程式已用于拟合测量或推导的属性。使用原位红外分析测量了石膏在ILs水溶液中的溶解动力学,并通过记录SEM图像检查了从各种条件获得的石膏晶体的形态。SEM图像显示了石膏的经典和非经典结晶行为,这取决于IL的性质。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号