Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crystal structures and calorimetry reveal catalytically relevant binding mode of coproporphyrin and coproheme in coproporphyrin ferrochelatase.
The FEBS Journal ( IF 5.5 ) Pub Date : 2019-12-03 , DOI: 10.1111/febs.15164 Stefan Hofbauer 1 , Johannes Helm 1 , Christian Obinger 1 , Kristina Djinović-Carugo 2, 3 , Paul G Furtmüller 1
The FEBS Journal ( IF 5.5 ) Pub Date : 2019-12-03 , DOI: 10.1111/febs.15164 Stefan Hofbauer 1 , Johannes Helm 1 , Christian Obinger 1 , Kristina Djinović-Carugo 2, 3 , Paul G Furtmüller 1
Affiliation
|
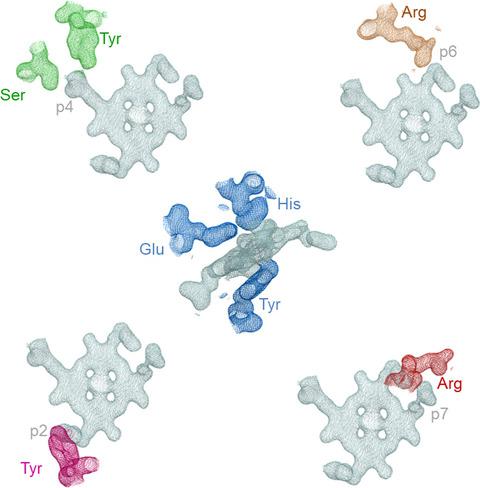
Coproporphyrin ferrochelatases (CpfCs, EC 4.99.1.9) insert ferrous iron into coproporphyrin III yielding coproheme. CpfCs are utilized by prokaryotic, mainly monoderm (Gram‐positive) bacteria within the recently detected coproporphyrin‐dependent (CPD) heme biosynthesis pathway. Here, we present a comprehensive study on CpfC from Listeria monocytogenes (Lm CpfC) including the first crystal structure of a coproheme‐bound CpfC. Comparison of crystal structures of apo‐Lm CpfC and coproheme‐Lm CpfC allowed identification of structural rearrangements and of amino acids involved in tetrapyrrole macrocycle and Fe2+ binding. Differential scanning calorimetry of apo‐, coproporphyrin III‐, and coproheme‐Lm CpfC underline the pronounced noncovalent interaction of both coproporphyrin and coproheme with the protein (ΔT m = 11 °C compared to apo‐Lm CpfC), which includes the propionates (p2, p4, p6, p7) and the amino acids Arg29, Arg45, Tyr46, Ser53, and Tyr124. Furthermore, the thermodynamics and kinetics of coproporphyrin III and coproheme binding to apo‐Lm CpfC is presented as well as the kinetics of insertion of ferrous iron into coproporphyrin III‐Lm CpfC that immediately leads to formation of ferric coproheme‐Lm CpfC (k cat/K M = 4.7 × 105 m −1·s−1). We compare the crystal structure of coproheme‐Lm CpfC with available structures of CpfCs with artificial tetrapyrrole macrocycles and discuss our data on substrate binding, iron insertion and substrate release in the context of the CPD heme biosynthesis pathway.
中文翻译:

晶体结构和量热法揭示了粪卟啉亚铁螯合酶中粪卟啉和粪血红素的催化相关结合模式。
粪卟啉亚铁螯合酶(CpfCs,EC 4.99.1.9)将亚铁插入粪卟啉 III 中,产生粪血红素。 CpfC 在最近检测到的粪卟啉依赖性 (CPD) 血红素生物合成途径中被原核、主要是单皮(革兰氏阳性)细菌利用。在这里,我们对单核细胞增生李斯特菌( Lm CpfC) 的 CpfC 进行了全面的研究,包括粪血红素结合的 CpfC 的第一个晶体结构。比较 apo- Lm CpfC 和 coproheme- Lm CpfC 的晶体结构,可以鉴定结构重排以及参与四吡咯大环和 Fe 2+结合的氨基酸。 apo-、粪卟啉 III- 和粪血红素Lm CpfC 的差示扫描量热法强调了粪卟啉和粪血红素与蛋白质之间明显的非共价相互作用(与 apo- Lm CpfC 相比,Δ T m = 11 °C),其中包括丙酸盐( p2、p4、p6、p7) 和氨基酸 Arg29、Arg45、Tyr46、Ser53 和 Tyr124。此外,还介绍了粪卟啉 III 和粪血红素与 apo -Lm CpfC 结合的热力学和动力学,以及将亚铁插入粪卟啉 III- Lm CpfC 中的动力学,立即导致三价铁粪血红素-Lm CpfC 的形成( k cat / K M = 4.7 × 10 5 m -1 ·s -1 )。 我们将 coproheme -Lm CpfC 的晶体结构与人工四吡咯大环化合物的 CpfC 的现有结构进行比较,并讨论了 CPD 血红素生物合成途径中底物结合、铁插入和底物释放的数据。
更新日期:2019-12-03
中文翻译:

晶体结构和量热法揭示了粪卟啉亚铁螯合酶中粪卟啉和粪血红素的催化相关结合模式。
粪卟啉亚铁螯合酶(CpfCs,EC 4.99.1.9)将亚铁插入粪卟啉 III 中,产生粪血红素。 CpfC 在最近检测到的粪卟啉依赖性 (CPD) 血红素生物合成途径中被原核、主要是单皮(革兰氏阳性)细菌利用。在这里,我们对单核细胞增生李斯特菌( Lm CpfC) 的 CpfC 进行了全面的研究,包括粪血红素结合的 CpfC 的第一个晶体结构。比较 apo- Lm CpfC 和 coproheme- Lm CpfC 的晶体结构,可以鉴定结构重排以及参与四吡咯大环和 Fe 2+结合的氨基酸。 apo-、粪卟啉 III- 和粪血红素Lm CpfC 的差示扫描量热法强调了粪卟啉和粪血红素与蛋白质之间明显的非共价相互作用(与 apo- Lm CpfC 相比,Δ T m = 11 °C),其中包括丙酸盐( p2、p4、p6、p7) 和氨基酸 Arg29、Arg45、Tyr46、Ser53 和 Tyr124。此外,还介绍了粪卟啉 III 和粪血红素与 apo -Lm CpfC 结合的热力学和动力学,以及将亚铁插入粪卟啉 III- Lm CpfC 中的动力学,立即导致三价铁粪血红素-Lm CpfC 的形成( k cat / K M = 4.7 × 10 5 m -1 ·s -1 )。 我们将 coproheme -Lm CpfC 的晶体结构与人工四吡咯大环化合物的 CpfC 的现有结构进行比较,并讨论了 CPD 血红素生物合成途径中底物结合、铁插入和底物释放的数据。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号