当前位置:
X-MOL 学术
›
Microchim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preconcentration of mercury(II) using a magnetite@carbon/dithizone nanocomposite, and its quantification by anodic stripping voltammetry
Microchimica Acta ( IF 5.3 ) Pub Date : 2019-12-03 , DOI: 10.1007/s00604-019-3937-0 Hossein Abdolmohammad-Zadeh 1 , Rahim Mohammad-Rezaei 1 , Arezu Salimi 1
Microchimica Acta ( IF 5.3 ) Pub Date : 2019-12-03 , DOI: 10.1007/s00604-019-3937-0 Hossein Abdolmohammad-Zadeh 1 , Rahim Mohammad-Rezaei 1 , Arezu Salimi 1
Affiliation
|
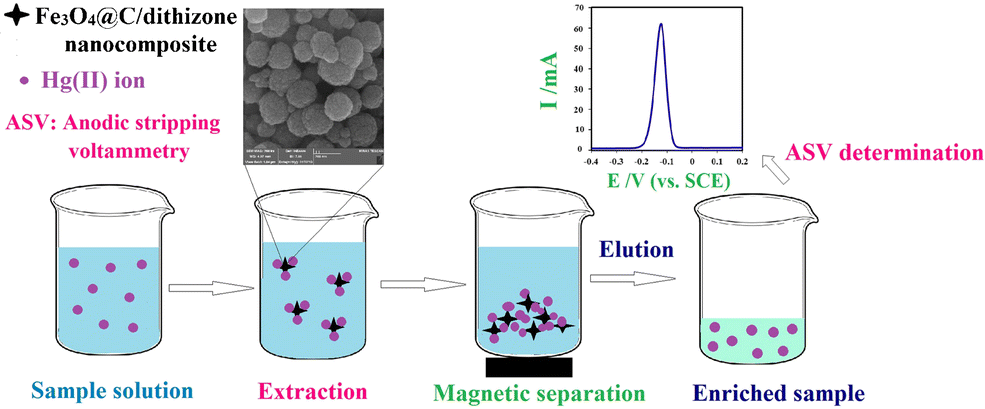
A new adsorbent is described that consists of a magnetite@carbon/dithizone nanocomposite. It was characterized using energy-dispersive X-ray spectroscopy, Fourier transform infrared spectroscopy, X-ray diffraction, and field emission scanning electron microscopy. The magnetic sorbent is shown to be a viable material for the preconcentration of mercury(II) before its quantification by differential pulse anodic stripping voltammetry. The effects of pH value, eluent, adsorbent amount, sample volume, and adsorption/desorption time were optimized. The calibration plot extends from 0.25 to 30 ng.mL−1, and the detection limit is 27 pg.mL−1. The preconcentration factor and intra-day and inter-day relative standard deviations are 100, 3.8, and 4.5%, respectively, for six measurements at 5 ng.mL−1 concentrations of mercury(II). The method was validated by the analysis of the certified reference material NIST SRM 1566b, and successfully applied to the preconcentration and quantification of mercury(II) in industrial wastewaters and spiked water samples. Graphical abstract Schematic representation of magnetic solid-phase extraction of mercury(II) ion by dithizone-modified Fe3O4@C nanocomposite (Fe3O4@C/Dz NC) before its quantification by anodic stripping voltammetry (ASV). Schematic representation of magnetic solid-phase extraction of mercury(II) ion by dithizone-modified Fe3O4@C nanocomposite (Fe3O4@C/Dz NC) before its quantification by anodic stripping voltammetry (ASV).
中文翻译:

使用磁铁矿@碳/双硫腙纳米复合材料预浓缩汞(II),并通过阳极溶出伏安法定量
描述了一种由磁铁矿@碳/双硫腙纳米复合材料组成的新型吸附剂。使用能量色散 X 射线光谱、傅里叶变换红外光谱、X 射线衍射和场发射扫描电子显微镜对其进行表征。在通过差分脉冲阳极溶出伏安法对其进行量化之前,磁性吸附剂被证明是一种用于预浓缩汞 (II) 的可行材料。对pH值、洗脱液、吸附剂用量、样品体积和吸附/解吸时间的影响进行了优化。校准曲线从 0.25 扩展到 30 ng.mL-1,检测限为 27 pg.mL-1。对于 5 ng.mL-1 汞 (II) 浓度下的六次测量,预浓缩因子和日内和日间相对标准偏差分别为 100、3.8 和 4.5%。该方法通过对有证标准物质 NIST SRM 1566b 的分析进行验证,并成功应用于工业废水和加标水样品中汞 (II) 的预浓缩和定量。图形摘要 在通过阳极溶出伏安法 (ASV) 量化之前,双硫腙修饰的 Fe3O4@C 纳米复合材料 (Fe3O4@C/Dz NC) 对汞 (II) 离子的磁性固相萃取示意图。在通过阳极溶出伏安法 (ASV) 量化之前,双硫腙修饰的 Fe3O4@C 纳米复合材料 (Fe3O4@C/Dz NC) 对汞 (II) 离子的磁性固相萃取示意图。图形摘要 在通过阳极溶出伏安法 (ASV) 定量之前,双硫腙修饰的 Fe3O4@C 纳米复合材料 (Fe3O4@C/Dz NC) 对汞 (II) 离子的磁性固相萃取示意图。在通过阳极溶出伏安法 (ASV) 定量之前,双硫腙修饰的 Fe3O4@C 纳米复合材料 (Fe3O4@C/Dz NC) 对汞 (II) 离子的磁性固相萃取示意图。图形摘要 在通过阳极溶出伏安法 (ASV) 定量之前,双硫腙修饰的 Fe3O4@C 纳米复合材料 (Fe3O4@C/Dz NC) 对汞 (II) 离子的磁性固相萃取示意图。在通过阳极溶出伏安法 (ASV) 定量之前,双硫腙修饰的 Fe3O4@C 纳米复合材料 (Fe3O4@C/Dz NC) 对汞 (II) 离子的磁性固相萃取示意图。
更新日期:2019-12-03
中文翻译:

使用磁铁矿@碳/双硫腙纳米复合材料预浓缩汞(II),并通过阳极溶出伏安法定量
描述了一种由磁铁矿@碳/双硫腙纳米复合材料组成的新型吸附剂。使用能量色散 X 射线光谱、傅里叶变换红外光谱、X 射线衍射和场发射扫描电子显微镜对其进行表征。在通过差分脉冲阳极溶出伏安法对其进行量化之前,磁性吸附剂被证明是一种用于预浓缩汞 (II) 的可行材料。对pH值、洗脱液、吸附剂用量、样品体积和吸附/解吸时间的影响进行了优化。校准曲线从 0.25 扩展到 30 ng.mL-1,检测限为 27 pg.mL-1。对于 5 ng.mL-1 汞 (II) 浓度下的六次测量,预浓缩因子和日内和日间相对标准偏差分别为 100、3.8 和 4.5%。该方法通过对有证标准物质 NIST SRM 1566b 的分析进行验证,并成功应用于工业废水和加标水样品中汞 (II) 的预浓缩和定量。图形摘要 在通过阳极溶出伏安法 (ASV) 量化之前,双硫腙修饰的 Fe3O4@C 纳米复合材料 (Fe3O4@C/Dz NC) 对汞 (II) 离子的磁性固相萃取示意图。在通过阳极溶出伏安法 (ASV) 量化之前,双硫腙修饰的 Fe3O4@C 纳米复合材料 (Fe3O4@C/Dz NC) 对汞 (II) 离子的磁性固相萃取示意图。图形摘要 在通过阳极溶出伏安法 (ASV) 定量之前,双硫腙修饰的 Fe3O4@C 纳米复合材料 (Fe3O4@C/Dz NC) 对汞 (II) 离子的磁性固相萃取示意图。在通过阳极溶出伏安法 (ASV) 定量之前,双硫腙修饰的 Fe3O4@C 纳米复合材料 (Fe3O4@C/Dz NC) 对汞 (II) 离子的磁性固相萃取示意图。图形摘要 在通过阳极溶出伏安法 (ASV) 定量之前,双硫腙修饰的 Fe3O4@C 纳米复合材料 (Fe3O4@C/Dz NC) 对汞 (II) 离子的磁性固相萃取示意图。在通过阳极溶出伏安法 (ASV) 定量之前,双硫腙修饰的 Fe3O4@C 纳米复合材料 (Fe3O4@C/Dz NC) 对汞 (II) 离子的磁性固相萃取示意图。































 京公网安备 11010802027423号
京公网安备 11010802027423号