Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and biological activities of two camptothecin derivatives against Spodoptera exigua.
Scientific Reports ( IF 3.8 ) Pub Date : 2019-12-02 , DOI: 10.1038/s41598-019-54596-y Fulai Yang 1, 2 , Liping Wang 1, 2 , Lan Zhang 1, 2 , Yanning Zhang 1, 2 , Liangang Mao 1, 2 , Hongyun Jiang 1, 2
Scientific Reports ( IF 3.8 ) Pub Date : 2019-12-02 , DOI: 10.1038/s41598-019-54596-y Fulai Yang 1, 2 , Liping Wang 1, 2 , Lan Zhang 1, 2 , Yanning Zhang 1, 2 , Liangang Mao 1, 2 , Hongyun Jiang 1, 2
Affiliation
|
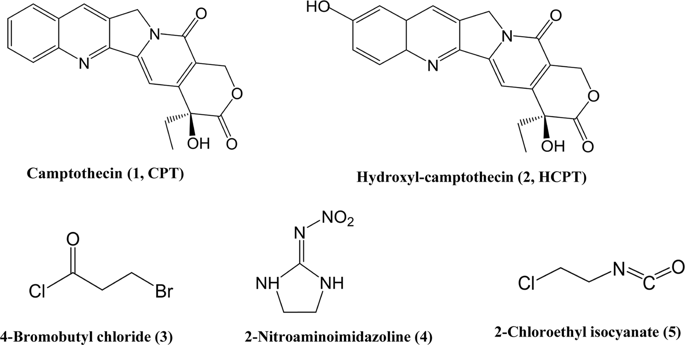
Camptothecin (CPT), a natural alkaloid isolated from Camptotheca acuminata Decne, is found to show potential insecticidal activities with unique action mechanisms by targeting at DNA-topoisomease I (Top1) complex and inducing cell apoptosis. To improve the efficacy against insect pests, two camptothecin (CPT) derivatives were synthesized through introducing two functional groups, 2-nitroaminoimidazoline and 1-chloro-2-isocyanatoethane by esterification reaction. The insecticidal activities of these two derivatives were evaluated at contact toxicity, cytotoxicity and topoisomerase I (Top1) inhibitory activities comparing with CPT and hydroxyl-camptothecin (HCPT). Results showed that compound a, synthesized by introducing 2-nitroaminoimidazoline to CPT, apparently increased contact toxicity to the third larvae of beet armyworm, Spodoptera exigua, and cytotoxicity to IOZCAS-Spex-II cells isolated from S. exigua. However, the inhibition on DNA relaxation activity of Top1 was reduced to less than 5 percentage even at high concentrations (50 and 100 μM). For introducing 1-chloro-2-isocyanatoethane to HCPT, the contact toxicity, cytotoxicity and Top1 inhibitory activity of synthesized compound b were increased significantly compared to CPT and HCPT. These results suggested that both synthesized compounds possessed high efficacy against S. exigua by targeting at Top1 (compound b) or novel mechanism of action (compound a).
中文翻译:

两种喜树碱衍生物对甜菜夜蛾的合成及生物活性。
喜树碱(CPT)是从喜树(Camptotheca acuminata Decne)分离的天然生物碱,通过靶向DNA-拓扑酶I(Top1)复合物并诱导细胞凋亡,显示出具有独特作用机制的潜在杀虫活性。为了提高对害虫的功效,通过酯化反应引入两个官能团2-硝基氨基咪唑啉和1-氯-2-异氰酸根合乙酯合成了两种喜树碱(CPT)衍生物。与CPT和羟基喜树碱(HCPT)相比,在接触毒性,细胞毒性和拓扑异构酶I(Top1)抑制活性方面评估了这两种衍生物的杀虫活性。结果表明,通过在CPT中引入2-硝基氨基咪唑啉合成的化合物a明显增加了对甜菜夜蛾甜菜夜蛾第三幼虫的接触毒性,对分离自S. exigua的IOZCAS-Spex-II细胞的毒性和细胞毒性 但是,即使在高浓度(50和100μM)下,对Top1的DNA松弛活性的抑制作用也降低到小于5%。为了将1-氯-2-异氰基乙烷引入HCPT,与CPT和HCPT相比,合成化合物b的接触毒性,细胞毒性和Top1抑制活性显着提高。这些结果表明,这两种合成的化合物都通过靶向Top1(化合物b)或新的作用机理(化合物a)而具有高抗S. exigua的功效。与CPT和HCPT相比,合成化合物b的细胞毒性和Top1抑制活性显着提高。这些结果表明,这两种合成的化合物都通过靶向Top1(化合物b)或新的作用机理(化合物a)而具有高抗S. exigua的功效。与CPT和HCPT相比,合成化合物b的细胞毒性和Top1抑制活性显着提高。这些结果表明,这两种合成的化合物都通过靶向Top1(化合物b)或新的作用机理(化合物a)而具有高抗S. exigua的功效。
更新日期:2019-12-02
中文翻译:

两种喜树碱衍生物对甜菜夜蛾的合成及生物活性。
喜树碱(CPT)是从喜树(Camptotheca acuminata Decne)分离的天然生物碱,通过靶向DNA-拓扑酶I(Top1)复合物并诱导细胞凋亡,显示出具有独特作用机制的潜在杀虫活性。为了提高对害虫的功效,通过酯化反应引入两个官能团2-硝基氨基咪唑啉和1-氯-2-异氰酸根合乙酯合成了两种喜树碱(CPT)衍生物。与CPT和羟基喜树碱(HCPT)相比,在接触毒性,细胞毒性和拓扑异构酶I(Top1)抑制活性方面评估了这两种衍生物的杀虫活性。结果表明,通过在CPT中引入2-硝基氨基咪唑啉合成的化合物a明显增加了对甜菜夜蛾甜菜夜蛾第三幼虫的接触毒性,对分离自S. exigua的IOZCAS-Spex-II细胞的毒性和细胞毒性 但是,即使在高浓度(50和100μM)下,对Top1的DNA松弛活性的抑制作用也降低到小于5%。为了将1-氯-2-异氰基乙烷引入HCPT,与CPT和HCPT相比,合成化合物b的接触毒性,细胞毒性和Top1抑制活性显着提高。这些结果表明,这两种合成的化合物都通过靶向Top1(化合物b)或新的作用机理(化合物a)而具有高抗S. exigua的功效。与CPT和HCPT相比,合成化合物b的细胞毒性和Top1抑制活性显着提高。这些结果表明,这两种合成的化合物都通过靶向Top1(化合物b)或新的作用机理(化合物a)而具有高抗S. exigua的功效。与CPT和HCPT相比,合成化合物b的细胞毒性和Top1抑制活性显着提高。这些结果表明,这两种合成的化合物都通过靶向Top1(化合物b)或新的作用机理(化合物a)而具有高抗S. exigua的功效。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号