当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and discovery of triazolo-pyridazine-6-yl-substituted piperazines as effective anti-diabetic drugs; evaluated over dipeptidyl peptidase-4 inhibition mechanism and insulinotropic activities.
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-11-30 , DOI: 10.1016/j.ejmech.2019.111912 B Bindu 1 , S Vijayalakshmi 1 , A Manikandan 2
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-11-30 , DOI: 10.1016/j.ejmech.2019.111912 B Bindu 1 , S Vijayalakshmi 1 , A Manikandan 2
Affiliation
|
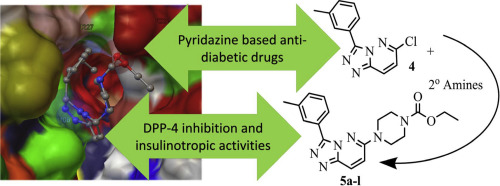
A family of 12 triazolo-pyridazine-6-yl-substituted piperazines (5a-l) was synthesized and evaluated for their Dipeptidyl peptidase-4 (DPP-4) inhibition potentials in order to develop them as anti-diabetic medications. In the two-step synthesis process, 6-chloro-3-(m-tolyl)-[1,2,4]triazolo[4,3-b]pyridazine was synthesized with one-pot mode using pyridine, 3,6-dichloropyridazine 5-(3-methyl-phenyl)tetrazole in toluene. Conjugating corresponding 2° amines with 6-chloro-3-(m-tolyl)-[1,2,4]triazolo[4,3-b]pyridazine afforded the target triazolo-pyridazine-6-yl-substituted piperazines (5a-l). DPP-4 inhibition potential of these compounds was testified in silico and in nitro along with their insulinotropic activities in 832/13 INS-1 cells. H2O2 radical scavenging assay and MTT assay were conducted to assess the antioxidant and cytotoxicity of these compounds respectively. Molecular docking and ELISA based enzyme inhibition assay results revealed the strong inhibition potential of the target compounds. MTT assay results indicated a maximum dose of 2.5 nM (IC50 1.25 nM) could be used and above this level vital for the cells. Compounds 5a, 5c, 5g and 5i were found with excellent antioxidant and insulinotropic activity up to 99%.
中文翻译:

三唑并哒嗪-6-基取代的哌嗪的合成及发现,作为有效的抗糖尿病药物;评估了二肽基肽酶-4的抑制机制和促胰岛素活性。
合成了十二族三唑并哒嗪-6-基取代的哌嗪(5a-1)家族,并对其二肽基肽酶-4(DPP-4)抑制潜力进行了评估,以便将其开发为抗糖尿病药物。在两步合成过程中,使用吡啶3,6-一锅法合成了6-氯-3-(间甲苯基)-[1,2,4]三唑并[4,3-b]哒嗪。二氯哒嗪5-(3-甲基-苯基)四唑的甲苯溶液。将相应的2°胺与6-氯-3-(间甲苯基)-[1,2,4]三唑并[4,3-b]哒嗪共轭可得到目标三唑并哒嗪-6-基取代的哌嗪(5a- l)。这些化合物对DPP-4的抑制潜力已在计算机和硝基中进行了验证,并在832/13 INS-1细胞中具有其促胰岛素活性。进行了H 2 O 2自由基清除测定和MTT测定,以分别评估这些化合物的抗氧化剂和细胞毒性。基于分子对接和ELISA的酶抑制试验结果表明目标化合物具有很强的抑制潜力。MTT分析结果表明,可以使用的最大剂量为2.5 nM(IC50 1.25 nM),超过此剂量对细胞至关重要。发现化合物5a,5c,5g和5i具有出色的抗氧化剂和促胰岛素活性,最高可达99%。
更新日期:2019-12-02
中文翻译:

三唑并哒嗪-6-基取代的哌嗪的合成及发现,作为有效的抗糖尿病药物;评估了二肽基肽酶-4的抑制机制和促胰岛素活性。
合成了十二族三唑并哒嗪-6-基取代的哌嗪(5a-1)家族,并对其二肽基肽酶-4(DPP-4)抑制潜力进行了评估,以便将其开发为抗糖尿病药物。在两步合成过程中,使用吡啶3,6-一锅法合成了6-氯-3-(间甲苯基)-[1,2,4]三唑并[4,3-b]哒嗪。二氯哒嗪5-(3-甲基-苯基)四唑的甲苯溶液。将相应的2°胺与6-氯-3-(间甲苯基)-[1,2,4]三唑并[4,3-b]哒嗪共轭可得到目标三唑并哒嗪-6-基取代的哌嗪(5a- l)。这些化合物对DPP-4的抑制潜力已在计算机和硝基中进行了验证,并在832/13 INS-1细胞中具有其促胰岛素活性。进行了H 2 O 2自由基清除测定和MTT测定,以分别评估这些化合物的抗氧化剂和细胞毒性。基于分子对接和ELISA的酶抑制试验结果表明目标化合物具有很强的抑制潜力。MTT分析结果表明,可以使用的最大剂量为2.5 nM(IC50 1.25 nM),超过此剂量对细胞至关重要。发现化合物5a,5c,5g和5i具有出色的抗氧化剂和促胰岛素活性,最高可达99%。






























 京公网安备 11010802027423号
京公网安备 11010802027423号