当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Polyamine Deacetylase Structure and Catalysis: Prokaryotic Acetylpolyamine Amidohydrolase and Eukaryotic HDAC10
Biochemistry ( IF 2.9 ) Pub Date : 2018-03-13 00:00:00 , DOI: 10.1021/acs.biochem.8b00079 Stephen A. Shinsky 1 , David W. Christianson 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-03-13 00:00:00 , DOI: 10.1021/acs.biochem.8b00079 Stephen A. Shinsky 1 , David W. Christianson 1
Affiliation
|
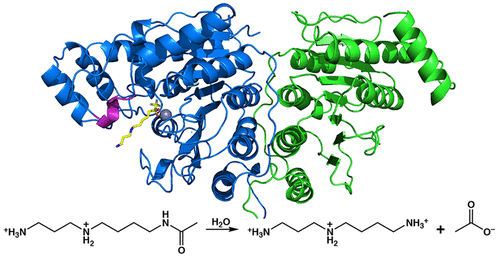
Polyamines such as putrescine, spermidine, and spermine are small aliphatic cations that serve myriad biological functions in all forms of life. While polyamine biosynthesis and cellular trafficking pathways are generally well-defined, only recently has the molecular basis of reversible polyamine acetylation been established. In particular, enzymes that catalyze polyamine deacetylation reactions have been identified and structurally characterized: histone deacetylase 10 (HDAC10) from Homo sapiens and Danio rerio (zebrafish) is a highly specific N8-acetylspermidine deacetylase, and its prokaryotic counterpart, acetylpolyamine amidohydrolase (APAH) from Mycoplana ramosa, is a broad-specificity polyamine deacetylase. Similar to the greater family of HDACs, which mainly serve as lysine deacetylases, both enzymes adopt the characteristic arginase-deacetylase fold and employ a Zn2+-activated water molecule for catalysis. In contrast with HDACs, however, the active sites of HDAC10 and APAH are sterically constricted to enforce specificity for long, slender polyamine substrates and exclude bulky peptides and proteins containing acetyl-l-lysine. Crystal structures of APAH and D. rerio HDAC10 reveal that quaternary structure, i.e., dimer assembly, provides the steric constriction that directs the polyamine substrate specificity of APAH, whereas tertiary structure, a unique 310 helix defined by the P(E,A)CE motif, provides the steric constriction that directs the polyamine substrate specificity of HDAC10. Given the recent identification of HDAC10 and spermidine as mediators of autophagy, HDAC10 is rapidly emerging as a biomarker and target for the design of isozyme-selective inhibitors that will suppress autophagic responses to cancer chemotherapy, thereby rendering cancer cells more susceptible to cytotoxic drugs.
中文翻译:

多胺脱乙酰酶的结构和催化:原核乙酰基聚胺酰胺水解酶和真核HDAC10
多胺(例如腐胺,亚精胺和精胺)是小的脂肪族阳离子,可在所有生命形式中发挥多种生物学功能。虽然多胺的生物合成和细胞运输途径通常是明确定义的,但直到最近才建立了可逆多胺乙酰化的分子基础。具体地,酶是催化脱乙酰多胺反应已被鉴定和结构表征:组蛋白脱乙酰10(HDAC10)从智人和斑马鱼(斑马鱼)是一种高度特异性的Ñ 8 -乙酰脱乙酰基酶,和它的原核对应,acetylpolyamine酰胺水解酶(APAH ),来自Mycoplana ramosa,是一种广泛特异性的多胺脱乙酰基酶。与主要用作赖氨酸脱乙酰基酶的更大的HDAC系列相似,这两种酶均采用特征性的精氨酸酶-脱乙酰基酶折叠,并使用Zn 2+活化的水分子进行催化。但是,与HDAC相比,HDAC10和APAH的活性位点在空间上受到限制,以增强对细长的多胺底物的特异性,并排除了大体积的肽和含乙酰基-1-赖氨酸的蛋白质。APAH和D. rerio HDAC10的晶体结构表明,四级结构(即二聚体组装)提供了空间收缩,可指导APAH的多胺底物特异性,而三级结构是唯一的3 10由P(E,A)CE基序定义的螺旋结构提供了指示HDAC10的多胺底物特异性的空间收缩。鉴于最近鉴定出HDAC10和亚精胺是自噬的介质,HDAC10迅速成为一种生物标志物,并成为设计同工酶选择性抑制剂的靶标,该抑制剂可抑制对癌症化学疗法的自噬反应,从而使癌细胞更易受细胞毒性药物的影响。
更新日期:2018-03-13
中文翻译:

多胺脱乙酰酶的结构和催化:原核乙酰基聚胺酰胺水解酶和真核HDAC10
多胺(例如腐胺,亚精胺和精胺)是小的脂肪族阳离子,可在所有生命形式中发挥多种生物学功能。虽然多胺的生物合成和细胞运输途径通常是明确定义的,但直到最近才建立了可逆多胺乙酰化的分子基础。具体地,酶是催化脱乙酰多胺反应已被鉴定和结构表征:组蛋白脱乙酰10(HDAC10)从智人和斑马鱼(斑马鱼)是一种高度特异性的Ñ 8 -乙酰脱乙酰基酶,和它的原核对应,acetylpolyamine酰胺水解酶(APAH ),来自Mycoplana ramosa,是一种广泛特异性的多胺脱乙酰基酶。与主要用作赖氨酸脱乙酰基酶的更大的HDAC系列相似,这两种酶均采用特征性的精氨酸酶-脱乙酰基酶折叠,并使用Zn 2+活化的水分子进行催化。但是,与HDAC相比,HDAC10和APAH的活性位点在空间上受到限制,以增强对细长的多胺底物的特异性,并排除了大体积的肽和含乙酰基-1-赖氨酸的蛋白质。APAH和D. rerio HDAC10的晶体结构表明,四级结构(即二聚体组装)提供了空间收缩,可指导APAH的多胺底物特异性,而三级结构是唯一的3 10由P(E,A)CE基序定义的螺旋结构提供了指示HDAC10的多胺底物特异性的空间收缩。鉴于最近鉴定出HDAC10和亚精胺是自噬的介质,HDAC10迅速成为一种生物标志物,并成为设计同工酶选择性抑制剂的靶标,该抑制剂可抑制对癌症化学疗法的自噬反应,从而使癌细胞更易受细胞毒性药物的影响。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号