当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gold-Catalyzed Intramolecular Dearomatization Reactions of Indoles for the Synthesis of Spiroindolenines and Spiroindolines.
Organic Letters ( IF 4.9 ) Pub Date : 2019-12-02 , DOI: 10.1021/acs.orglett.9b03988 Wen-Ting Wu 1 , Lu Ding 2 , Liming Zhang 3 , Shu-Li You 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2019-12-02 , DOI: 10.1021/acs.orglett.9b03988 Wen-Ting Wu 1 , Lu Ding 2 , Liming Zhang 3 , Shu-Li You 1, 2
Affiliation
|
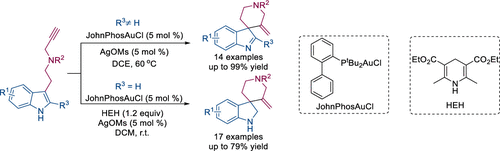
A gold-catalyzed dearomatization reaction of indole derivatives was realized in the presence of JohnPhosAuCl/AgOMs to afford a series of spiroindolenines in excellent yields (≤99%). In addition, when the Hantzsch ester was used as the hydrogen transfer reagent, various spiroindolines were obtained in a cascade fashion starting from readily available indole derivatives in modest to good yields (≤79%). Both reactions feature readily available substrates, mild conditions, and good functional group tolerance.
中文翻译:

吲哚的金催化的分子内脱芳香化反应,用于合成螺吲哚啉和螺吲哚啉。
在JohnPhosAuCl / AgOMs的存在下实现了金催化的吲哚衍生物的脱芳香化反应,从而以优异的收率(≤99%)提供了一系列螺环吲哚胺。另外,当使用汉茨sch酯作为氢转移试剂时,从容易获得的吲哚衍生物以适度到良好的收率(≤79%)以级联方式获得了各种螺二氢吲哚。两种反应均具有易于获得的底物,温和的条件和良好的官能团耐受性。
更新日期:2019-12-02
中文翻译:

吲哚的金催化的分子内脱芳香化反应,用于合成螺吲哚啉和螺吲哚啉。
在JohnPhosAuCl / AgOMs的存在下实现了金催化的吲哚衍生物的脱芳香化反应,从而以优异的收率(≤99%)提供了一系列螺环吲哚胺。另外,当使用汉茨sch酯作为氢转移试剂时,从容易获得的吲哚衍生物以适度到良好的收率(≤79%)以级联方式获得了各种螺二氢吲哚。两种反应均具有易于获得的底物,温和的条件和良好的官能团耐受性。































 京公网安备 11010802027423号
京公网安备 11010802027423号