当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Total Synthesis of Pre-schisanartanin C
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-12-02 , DOI: 10.1021/jacs.9b11872 Yan-Long Jiang 1 , Hai-Xin Yu 1 , Yong Li 1 , Pei Qu 1 , Yi-Xin Han 1 , Jia-Hua Chen 1 , Zhen Yang 1, 2, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-12-02 , DOI: 10.1021/jacs.9b11872 Yan-Long Jiang 1 , Hai-Xin Yu 1 , Yong Li 1 , Pei Qu 1 , Yi-Xin Han 1 , Jia-Hua Chen 1 , Zhen Yang 1, 2, 3
Affiliation
|
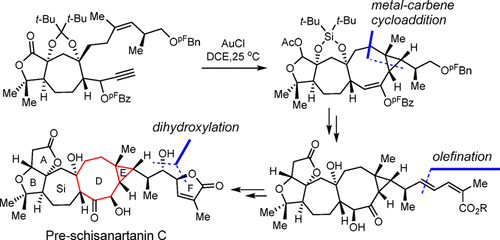
Pre-schisanartanin C belongs to the family of Schisandra nortriterpenoids with potent antihepatitis, antitumor, and anti-HIV activities. This paper presents the enantioselective total synthesis of pre-schisanartanin C (1). An important step in the total synthesis of 1 is gold-catalyzed intramolecular cyclopropanation of an 1,8-enyne substrates bearing a secondary ester group at the propargylic position to prepare a bicyclo[6.1.0]nonane core. Additional highlights include i) an asymmetric Diels-Alder reaction to install the initial C5 stereogenic center of 1, and ii) a sequential Pd-catalyzed Stille coupling, regio- and stereo-selective Sharpless asymmetric dihydroxylation, and a subsequent intramolecular lactonization to contruct the side chain of 1. The developed chemistry paves the way for the total syntheses of other family members bearing highly rigid bicyclo[6.1.0]nonane cores.
中文翻译:

前五味子素C的不对称全合成
五味子素前体 C 属于五味子降三萜类化合物家族,具有有效的抗肝炎、抗肿瘤和抗 HIV 活性。本文介绍了前五味子素 C (1) 的对映选择性全合成。1 的全合成中的一个重要步骤是金催化的分子内环丙烷化,在炔丙基位置带有仲酯基的 1,8-烯炔底物制备双环 [6.1.0] 壬烷核。其他亮点包括 i) 不对称 Diels-Alder 反应以安装 1 的初始 C5 立体中心,以及 ii) 顺序 Pd 催化的 Stille 偶联、区域和立体选择性 Sharpless 不对称二羟基化,以及随后的分子内内酯化以构建1的侧链
更新日期:2019-12-02
中文翻译:

前五味子素C的不对称全合成
五味子素前体 C 属于五味子降三萜类化合物家族,具有有效的抗肝炎、抗肿瘤和抗 HIV 活性。本文介绍了前五味子素 C (1) 的对映选择性全合成。1 的全合成中的一个重要步骤是金催化的分子内环丙烷化,在炔丙基位置带有仲酯基的 1,8-烯炔底物制备双环 [6.1.0] 壬烷核。其他亮点包括 i) 不对称 Diels-Alder 反应以安装 1 的初始 C5 立体中心,以及 ii) 顺序 Pd 催化的 Stille 偶联、区域和立体选择性 Sharpless 不对称二羟基化,以及随后的分子内内酯化以构建1的侧链































 京公网安备 11010802027423号
京公网安备 11010802027423号