Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural and functional role of disulphide bonds and substrate binding residues of the human beta-galactoside alpha-2,3-sialyltransferase 1 (hST3Gal1).
Scientific Reports ( IF 3.8 ) Pub Date : 2019-11-29 , DOI: 10.1038/s41598-019-54384-8 Maria Elena Ortiz-Soto 1 , Sabine Reising 1 , Andreas Schlosser 2 , Jürgen Seibel 1
Scientific Reports ( IF 3.8 ) Pub Date : 2019-11-29 , DOI: 10.1038/s41598-019-54384-8 Maria Elena Ortiz-Soto 1 , Sabine Reising 1 , Andreas Schlosser 2 , Jürgen Seibel 1
Affiliation
|
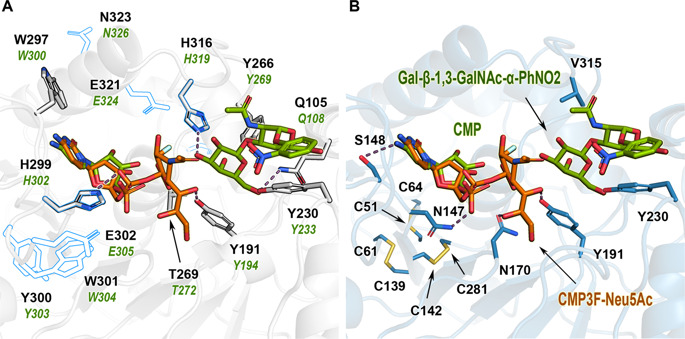
Overexpression of hST3Gal1 leads to hypersialylation of cell-surface glycoconjugates, a cancer-associated condition that promotes cell growth, migration and invasion. Upregulation of this enzyme in ovarian cancer is linked to cancer progression and metastasis, contributing also to chemotherapy resistance. Strategies for preventing metastasis include the inhibition of hST3Gal1, which demands structure-based studies on its strict regioselectivity and substrate/donor preference. Herein we describe the contribution of various residues constituting donor CMP-Neu5Ac and acceptor Galβ1-3GalNAc-R binding sites to catalysis. Removal of hydrogen bonds and/or stacking interactions among substrates and residues Y191, Y230, N147, S148 and N170 affected the enzyme's activity to a different extent, revealing the fine control needed for an optimal catalytic performance. To gain further understanding of the correlation among structure, activity and stability, the in vitro role of hST3Gal1 disulphide bonds was analysed. As expected, disruption of the Glycosyltransferase family 29 (GT29) invariant bond C142-C281, as well as the ST3Gal1 subfamily conserved disulphide C61-C139 inactivates the enzyme. While disulphide C59-C64 is not essential for function, its absence reduces the activity (kcat) for donor and acceptor substrates to about 67 and 72%, respectively, and diminishes the enzyme's melting temperature (Tm) by 7 °C.
中文翻译:

人β-半乳糖苷α-2,3-唾液酸转移酶1(hST3Gal1)的二硫键和底物结合残基的结构和功能作用。
hST3Gal1的过表达导致细胞表面糖缀合物的过度唾液酸化,这是一种与癌症相关的病症,可促进细胞生长,迁移和侵袭。卵巢癌中这种酶的上调与癌症的进展和转移有关,也对化疗耐药性有贡献。预防转移的策略包括抑制hST3Gal1,这需要对其严格的区域选择性和底物/供体的偏好进行基于结构的研究。在本文中,我们描述了构成供体CMP-Neu5Ac和受体Galβ1-3GalNAc-R结合位点的各种残基对催化的贡献。底物与残基Y191,Y230,N147,S148和N170之间的氢键去除和/或堆积相互作用在不同程度上影响了酶的活性,揭示了最佳催化性能所需的精细控制。为了进一步了解结构,活性和稳定性之间的相关性,分析了hST3Gal1二硫键的体外作用。正如预期的那样,糖基转移酶家族29(GT29)不变键C142-C281的破坏,以及ST3Gal1亚家族保守的二硫化物C61-C139均使该酶失活。尽管二硫化物C59-C64对功能不是必需的,但不存在二硫化物C59-C64时,供体和受体底物的活性(kcat)分别降低至约67%和72%,并使酶的解链温度(Tm)降低7°C。糖基转移酶家族29(GT29)不变键C142-C281的破坏,以及ST3Gal1亚家族保守的二硫键C61-C139使该酶失活。尽管二硫化物C59-C64对功能不是必需的,但缺少二硫化物C59-C64会使供体和受体底物的活性(kcat)分别降低至约67%和72%,并使酶的解链温度(Tm)降低7°C。糖基转移酶家族29(GT29)不变键C142-C281的破坏,以及ST3Gal1亚家族保守的二硫化物C61-C139使该酶失活。尽管二硫化物C59-C64对功能不是必需的,但不存在二硫化物C59-C64时,供体和受体底物的活性(kcat)分别降低至约67%和72%,并使酶的解链温度(Tm)降低7°C。
更新日期:2019-11-30
中文翻译:

人β-半乳糖苷α-2,3-唾液酸转移酶1(hST3Gal1)的二硫键和底物结合残基的结构和功能作用。
hST3Gal1的过表达导致细胞表面糖缀合物的过度唾液酸化,这是一种与癌症相关的病症,可促进细胞生长,迁移和侵袭。卵巢癌中这种酶的上调与癌症的进展和转移有关,也对化疗耐药性有贡献。预防转移的策略包括抑制hST3Gal1,这需要对其严格的区域选择性和底物/供体的偏好进行基于结构的研究。在本文中,我们描述了构成供体CMP-Neu5Ac和受体Galβ1-3GalNAc-R结合位点的各种残基对催化的贡献。底物与残基Y191,Y230,N147,S148和N170之间的氢键去除和/或堆积相互作用在不同程度上影响了酶的活性,揭示了最佳催化性能所需的精细控制。为了进一步了解结构,活性和稳定性之间的相关性,分析了hST3Gal1二硫键的体外作用。正如预期的那样,糖基转移酶家族29(GT29)不变键C142-C281的破坏,以及ST3Gal1亚家族保守的二硫化物C61-C139均使该酶失活。尽管二硫化物C59-C64对功能不是必需的,但不存在二硫化物C59-C64时,供体和受体底物的活性(kcat)分别降低至约67%和72%,并使酶的解链温度(Tm)降低7°C。糖基转移酶家族29(GT29)不变键C142-C281的破坏,以及ST3Gal1亚家族保守的二硫键C61-C139使该酶失活。尽管二硫化物C59-C64对功能不是必需的,但缺少二硫化物C59-C64会使供体和受体底物的活性(kcat)分别降低至约67%和72%,并使酶的解链温度(Tm)降低7°C。糖基转移酶家族29(GT29)不变键C142-C281的破坏,以及ST3Gal1亚家族保守的二硫化物C61-C139使该酶失活。尽管二硫化物C59-C64对功能不是必需的,但不存在二硫化物C59-C64时,供体和受体底物的活性(kcat)分别降低至约67%和72%,并使酶的解链温度(Tm)降低7°C。































 京公网安备 11010802027423号
京公网安备 11010802027423号