当前位置:
X-MOL 学术
›
J. Colloid Interface Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Experimental and computational studies onpropanone derivatives of quinoxalin-6-yl-4,5-dihydropyrazole as inhibitors of mild steel corrosion in hydrochloric acid.
Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2019-11-29 , DOI: 10.1016/j.jcis.2019.11.097 Lukman O Olasunkanmi 1 , Eno E Ebenso 2
Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2019-11-29 , DOI: 10.1016/j.jcis.2019.11.097 Lukman O Olasunkanmi 1 , Eno E Ebenso 2
Affiliation
|
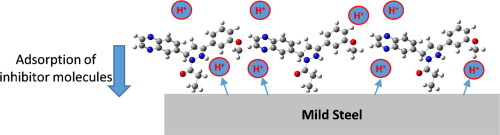
Two quinoxaline-based propanones, 1-[3-(3-methoxyphenyl)-5-(quinoxalin-6-yl)-4,5-dihydropyrazol-1-yl]propan-1-one (Mt-3-PQPP) and 1-(3-(4-chlorophenyl)-5-(quinoxalin-6-yl)-4,5-dihydro-1H-pyrazol-1-yl)propan-1-one (Cl-4-PQPP) were tested as inhibitors of mild steel corrosion in 1 M HCl using both experimental and computational approaches. Both compounds were found to retard corrosion rate of mild steel in the studied medium. Mt-3-PQPP and Cl-4-PQPP exhibited mixed-type inhibitive action, reducing the rate of anodic and cathodic corrosion reactions, as suggested by Tafel polarization measurements. Adsorbed molecules of Mt-3-PQPP and Cl-4-PQPP formed pseudo-capacitive film on mild steel surface in 1 M HCl as proposed by electrochemical impedance spectroscopy (EIS) measurements. Adsorption surface coverage data were fitted into the Langmuir adsorption isotherm and the evaluated thermodynamic parameters suggested chemisorption for Mt-3-PQPP and competitive physisorption and chemisorption for Cl-4-PQPP. Scanning electron microscopy (SEM) analyses further revealed that adsorbed film of the inhibitor molecules protected the steel from direct exposure to acidic ions. Quantum chemical calculations suggested that higher corrosion inhibition efficiency of Mt-3-PQPP compared to Cl-4-PQPP molecule is due to the higher electron donating tendency of the former. Mt-3-PQPP molecule also showed higher protonation tendency in the acid than Cl-4-PQPP and its protonated form showed better corrosion inhibition potentials than that of Cl-4-PQPP. Monte Carlo simulation of the adsorption of Mt-3-PQPP and Cl-4-PQPP molecules on Fe(1 1 0) surface also confirmed higher adsorption energy for the former.
中文翻译:

喹喔啉-6-基-4,5-二氢吡唑的丙酮衍生物作为缓蚀剂在盐酸中的缓蚀剂的实验和计算研究。
两个基于喹喔啉的丙烷,1- [3-(3-甲氧基苯基)-5-(喹喔啉-6-基)-4,5-二氢吡唑-1-基]丙-1-酮(Mt-3-PQPP)和测试了1-(3-(4-氯苯基)-5-(喹喔啉-6-基)-4,5-二氢-1H-吡唑-1-基)丙-1-酮(Cl-4-PQPP)使用实验和计算方法在1 M HCl中抑制低碳钢腐蚀。发现这两种化合物都可在所研究的介质中延缓低碳钢的腐蚀速率。Tafel极化测量表明,Mt-3-PQPP和Cl-4-PQPP表现出混合型抑制作用,降低了阳极和阴极腐蚀反应的速率。Mt-3-PQPP和Cl-4-PQPP的吸附分子在1 M HCl中在低碳钢表面上形成伪电容膜,这是通过电化学阻抗谱(EIS)测量提出的。将吸附表面覆盖数据拟合到Langmuir吸附等温线中,评估的热力学参数表明Mt-3-PQPP的化学吸附和Cl-4-PQPP的竞争性物理吸附和化学吸附。扫描电子显微镜(SEM)分析进一步表明,抑制剂分子的吸附膜可保护钢免于直接暴露于酸性离子。量子化学计算表明,与Cl-4-PQPP分子相比,Mt-3-PQPP的腐蚀抑制效率更高,这是由于前者具有更高的供电子趋势。Mt-3-PQPP分子在酸中也显示出比Cl-4-PQPP更高的质子化趋势,并且其质子化形式显示出比Cl-4-PQPP更好的缓蚀潜能。
更新日期:2019-11-29
中文翻译:

喹喔啉-6-基-4,5-二氢吡唑的丙酮衍生物作为缓蚀剂在盐酸中的缓蚀剂的实验和计算研究。
两个基于喹喔啉的丙烷,1- [3-(3-甲氧基苯基)-5-(喹喔啉-6-基)-4,5-二氢吡唑-1-基]丙-1-酮(Mt-3-PQPP)和测试了1-(3-(4-氯苯基)-5-(喹喔啉-6-基)-4,5-二氢-1H-吡唑-1-基)丙-1-酮(Cl-4-PQPP)使用实验和计算方法在1 M HCl中抑制低碳钢腐蚀。发现这两种化合物都可在所研究的介质中延缓低碳钢的腐蚀速率。Tafel极化测量表明,Mt-3-PQPP和Cl-4-PQPP表现出混合型抑制作用,降低了阳极和阴极腐蚀反应的速率。Mt-3-PQPP和Cl-4-PQPP的吸附分子在1 M HCl中在低碳钢表面上形成伪电容膜,这是通过电化学阻抗谱(EIS)测量提出的。将吸附表面覆盖数据拟合到Langmuir吸附等温线中,评估的热力学参数表明Mt-3-PQPP的化学吸附和Cl-4-PQPP的竞争性物理吸附和化学吸附。扫描电子显微镜(SEM)分析进一步表明,抑制剂分子的吸附膜可保护钢免于直接暴露于酸性离子。量子化学计算表明,与Cl-4-PQPP分子相比,Mt-3-PQPP的腐蚀抑制效率更高,这是由于前者具有更高的供电子趋势。Mt-3-PQPP分子在酸中也显示出比Cl-4-PQPP更高的质子化趋势,并且其质子化形式显示出比Cl-4-PQPP更好的缓蚀潜能。






































 京公网安备 11010802027423号
京公网安备 11010802027423号