Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
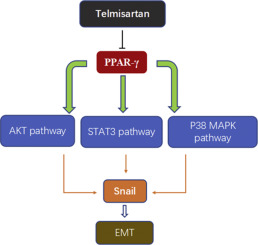
Telmisartan inhibits oxalate and calcium oxalate crystal-induced epithelial-mesenchymal transformation via PPAR-γ-AKT/STAT3/p38 MAPK-Snail pathway.
Life Sciences ( IF 5.2 ) Pub Date : 2019-11-28 , DOI: 10.1016/j.lfs.2019.117108 Yadong Liu 1 , Song Chen 1 , Jiannan Liu 1 , Yinshan Jin 1 , Shiliang Yu 1 , Ruihua An 1
Life Sciences ( IF 5.2 ) Pub Date : 2019-11-28 , DOI: 10.1016/j.lfs.2019.117108 Yadong Liu 1 , Song Chen 1 , Jiannan Liu 1 , Yinshan Jin 1 , Shiliang Yu 1 , Ruihua An 1
Affiliation
|
AIMS
Telmisartan (TLM), a highly selective angiotensin II type 1 receptor blocker (ARB) and partial PPAR-γ agonist, has versatile beneficial effects against oxidative stress, apoptosis, inflammatory responses and epithelial-mesenchymal transition (EMT). However, its underlying mechanism of inhibiting oxalate and calcium oxalate (CaOx) crystal-induced EMT by activating the PPAR-γ pathway remains unclear.
MAIN METHODS
CCK-8 assays were used to evaluate the effects of TLM on cell viability. In addition, intracellular reactive oxygen species (ROS) levels were measured by the cell-permeable fluorogenic probe 2,7-dichlorofluorescein diacetate (DCFH-DA). Wound-healing and Transwell assays were used to evaluate the migration ability of HK2 cells exposed to oxalate. Moreover, immunofluorescence, immunohistochemistry and western blotting were used to examine the expression of E-cadherin, N-cadherin, vimentin and α-SMA and explore the underlying molecular mechanisms in HK2 cells and a stone-forming rat model.
KEY FINDINGS
Our results showed that TLM treatment could protect HK2 cells from oxalate-induced cytotoxicity and oxidative stress injury. Additionally, TLM prevented EMT induction by oxalate and CaOx crystals via the PPAR-γ-AKT/STAT3/p38 MAPK-Snail pathway in vitro and in vivo. However, knockdown of PPAR-γ with small interfering RNA or the PPAR-γ-specific antagonist GW9662 abrogated these protective effects of TLM.
SIGNIFICANCE
As a PPAR-γ agonist, TLM can ameliorate oxalate and CaOx crystal-induced EMT by exerting an antioxidant effect through the PPAR-γ-AKT/STAT3/p38 MAPK-Snail signaling pathway. Therefore, TLM can block EMT progression and could be a potential therapeutic agent for preventing and treating calcium oxalate urolithiasis formation and recurrence.
中文翻译:

Telmisartan 通过 PPAR-γ-AKT/STAT3/p38 MAPK-Snail 通路抑制草酸盐和草酸钙晶体诱导的上皮-间质转化。
目的 替米沙坦 (TLM) 是一种高选择性血管紧张素 II 1 型受体阻滞剂 (ARB) 和部分 PPAR-γ 激动剂,对氧化应激、细胞凋亡、炎症反应和上皮-间质转化 (EMT) 具有多种有益作用。然而,其通过激活 PPAR-γ 通路抑制草酸盐和草酸钙 (CaOx) 晶体诱导的 EMT 的潜在机制仍不清楚。主要方法 CCK-8 检测用于评估 TLM 对细胞活力的影响。此外,细胞内活性氧 (ROS) 水平通过细胞渗透性荧光探针 2,7-二氯荧光素二乙酸酯 (DCFH-DA) 测量。伤口愈合和 Transwell 测定用于评估暴露于草酸盐的 HK2 细胞的迁移能力。此外,免疫荧光,采用免疫组织化学和蛋白质印迹法检测 E-钙粘蛋白、N-钙粘蛋白、波形蛋白和 α-SMA 的表达,并探索 HK2 细胞和结石形成大鼠模型的潜在分子机制。主要发现 我们的结果表明,TLM 处理可以保护 HK2 细胞免受草酸盐诱导的细胞毒性和氧化应激损伤。此外,TLM 在体外和体内通过 PPAR-γ-AKT/STAT3/p38 MAPK-Snail 通路阻止草酸盐和 CaOx 晶体诱导 EMT。然而,用小干扰 RNA 或 PPAR-γ 特异性拮抗剂 GW9662 敲低 PPAR-γ 可消除 TLM 的这些保护作用。意义 作为 PPAR-γ 激动剂,TLM 可以通过 PPAR-γ-AKT/STAT3/p38 MAPK-Snail 信号通路发挥抗氧化作用,从而改善草酸盐和 CaOx 晶体诱导的 EMT。所以,
更新日期:2019-11-29
中文翻译:

Telmisartan 通过 PPAR-γ-AKT/STAT3/p38 MAPK-Snail 通路抑制草酸盐和草酸钙晶体诱导的上皮-间质转化。
目的 替米沙坦 (TLM) 是一种高选择性血管紧张素 II 1 型受体阻滞剂 (ARB) 和部分 PPAR-γ 激动剂,对氧化应激、细胞凋亡、炎症反应和上皮-间质转化 (EMT) 具有多种有益作用。然而,其通过激活 PPAR-γ 通路抑制草酸盐和草酸钙 (CaOx) 晶体诱导的 EMT 的潜在机制仍不清楚。主要方法 CCK-8 检测用于评估 TLM 对细胞活力的影响。此外,细胞内活性氧 (ROS) 水平通过细胞渗透性荧光探针 2,7-二氯荧光素二乙酸酯 (DCFH-DA) 测量。伤口愈合和 Transwell 测定用于评估暴露于草酸盐的 HK2 细胞的迁移能力。此外,免疫荧光,采用免疫组织化学和蛋白质印迹法检测 E-钙粘蛋白、N-钙粘蛋白、波形蛋白和 α-SMA 的表达,并探索 HK2 细胞和结石形成大鼠模型的潜在分子机制。主要发现 我们的结果表明,TLM 处理可以保护 HK2 细胞免受草酸盐诱导的细胞毒性和氧化应激损伤。此外,TLM 在体外和体内通过 PPAR-γ-AKT/STAT3/p38 MAPK-Snail 通路阻止草酸盐和 CaOx 晶体诱导 EMT。然而,用小干扰 RNA 或 PPAR-γ 特异性拮抗剂 GW9662 敲低 PPAR-γ 可消除 TLM 的这些保护作用。意义 作为 PPAR-γ 激动剂,TLM 可以通过 PPAR-γ-AKT/STAT3/p38 MAPK-Snail 信号通路发挥抗氧化作用,从而改善草酸盐和 CaOx 晶体诱导的 EMT。所以,






























 京公网安备 11010802027423号
京公网安备 11010802027423号