Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An electrochemical study of pH influences on corrosion and passivation for a Q235 carbon steel in HNO3–NaNO2, HAc–NaNO2 and HCl–NaNO2 solutions
RSC Advances ( IF 3.9 ) Pub Date : 2019-11-28 , DOI: 10.1039/c9ra08482g
Xuan Li 1 , Pei Zhang 2 , Huiju Huang 1 , Xiaochen Hu 1 , Yong Zhou 1 , Fuan Yan 1
RSC Advances ( IF 3.9 ) Pub Date : 2019-11-28 , DOI: 10.1039/c9ra08482g
Xuan Li 1 , Pei Zhang 2 , Huiju Huang 1 , Xiaochen Hu 1 , Yong Zhou 1 , Fuan Yan 1
Affiliation
|
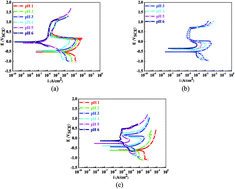
In this study, the influences of different pH values on the corrosion and passivation behaviors of a Q235 carbon steel in HNO3–NaNO2, HAc–NaNO2 and HCl–NaNO2 solutions were studied by electrochemical methods. The manifestations of the electrochemical characteristics were revealed and the variations in the electrochemical parameters were clarified. Moreover, for the Q235 steel in the three solutions with different pH values, the decrease in the corrosion current density (icorr) and the increase in the charge transfer resistance (Rct) in each solution, indicated a decrease in the corrosion rate. The decrease in the critical passivation current density (icrit) and increase in the passive film resistance (Rf) suggested the reinforcement of passivation capability. On the other hand, in the three solutions at the same pH value, the corrosion rate increased and the passivation capability weakened in HNO3–NaNO2, HAc–NaNO2 and HCl–NaNO2 solutions. Simultaneously, the related electrochemical mechanisms of corrosion and passivation for Q235 carbon steel in acidic solutions containing nitrite anions (NO2−) were also discussed.
中文翻译:

电化学研究 pH 对 Q235 碳钢在 HNO3-NaNO2、HAc-NaNO2 和 HCl-NaNO2 溶液中腐蚀和钝化的影响
本研究采用电化学方法研究了不同pH值对Q235碳钢在HNO 3 -NaNO 2、HAc-NaNO 2和HCl-NaNO 2溶液中腐蚀和钝化行为的影响。揭示了电化学特性的表现,阐明了电化学参数的变化。此外,对于 Q235 钢,在不同 pH 值的三种溶液中,腐蚀电流密度 ( i corr ) 的降低和电荷转移电阻 ( R ct ) 的增加表明腐蚀速率有所降低。临界钝化电流密度的降低(i crit ) 和钝化膜电阻 ( R f ) 的增加表明钝化能力增强。另一方面,在相同pH值的三种溶液中,HNO 3 -NaNO 2、HAc-NaNO 2和HCl-NaNO 2溶液的腐蚀速率增加,钝化能力减弱。同时,还讨论了Q235碳钢在含有亚硝酸根阴离子(NO 2 - )的酸性溶液中腐蚀和钝化的相关电化学机制。
更新日期:2019-11-29
中文翻译:

电化学研究 pH 对 Q235 碳钢在 HNO3-NaNO2、HAc-NaNO2 和 HCl-NaNO2 溶液中腐蚀和钝化的影响
本研究采用电化学方法研究了不同pH值对Q235碳钢在HNO 3 -NaNO 2、HAc-NaNO 2和HCl-NaNO 2溶液中腐蚀和钝化行为的影响。揭示了电化学特性的表现,阐明了电化学参数的变化。此外,对于 Q235 钢,在不同 pH 值的三种溶液中,腐蚀电流密度 ( i corr ) 的降低和电荷转移电阻 ( R ct ) 的增加表明腐蚀速率有所降低。临界钝化电流密度的降低(i crit ) 和钝化膜电阻 ( R f ) 的增加表明钝化能力增强。另一方面,在相同pH值的三种溶液中,HNO 3 -NaNO 2、HAc-NaNO 2和HCl-NaNO 2溶液的腐蚀速率增加,钝化能力减弱。同时,还讨论了Q235碳钢在含有亚硝酸根阴离子(NO 2 - )的酸性溶液中腐蚀和钝化的相关电化学机制。

































 京公网安备 11010802027423号
京公网安备 11010802027423号