当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
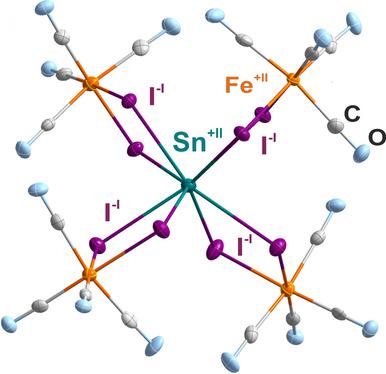
[SnI8 {Fe(CO)4 }4 ]2+ : Highly Coordinated Sn+II I8 Subunit with Fragile Carbonyl Clips.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-11-27 , DOI: 10.1002/anie.201911126 Silke Wolf 1 , Ralf Köppe 1 , Theresa Block 2 , Rainer Pöttgen 2 , Peter W Roesky 1 , Claus Feldmann 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-11-27 , DOI: 10.1002/anie.201911126 Silke Wolf 1 , Ralf Köppe 1 , Theresa Block 2 , Rainer Pöttgen 2 , Peter W Roesky 1 , Claus Feldmann 1
Affiliation
|
[SnI8 {Fe(CO)4 }4 ][Al2 Cl7 ]2 contains the [SnI8 {Fe(CO)4 }4 ]2+ cation with an unprecedented highly coordinated, bicapped SnI8 prism. Given the eightfold coordination with the most voluminous stable halide, it is all the more surprising that this SnI8 arrangement is surrounded only by fragile Fe(CO)4 groups in a clip-like fashion. Inspite of a predominantly ionic bonding situation in [SnI8 {Fe(CO)4 }4 ]2+ , the I- ⋅⋅⋅I- distances are considerably shortened (down to 371 pm) and significantly less than the van der Waals distance (420 pm). The title compound is characterized by single-crystal structure analysis, spectroscopic methods (EDXS, FTIR, Raman, UV/Vis, Mössbauer), thermogravimetry, and density functional theory methods.
中文翻译:

[SnI8 {Fe(CO)4} 4] 2+:具有易碎羰基夹的高度配位的Sn + II I8亚基。
[SnI8 {Fe(CO)4} 4] [Al2 Cl7] 2包含[SnI8 {Fe(CO)4} 4] 2+阳离子,该阳离子具有空前的高度配位的,二配位的SnI8棱镜。考虑到与最大量的稳定卤化物的八重配合,更令人惊讶的是,这种SnI8排列仅以易碎的Fe(CO)4基团以夹子状的方式包围。尽管在[SnI8 {Fe(CO)4} 4] 2+中离子键占主导地位,但I-⋅⋅⋅I-距离明显缩短(低至371 pm),并且显着小于范德华距离( 420 pm)。标题化合物的特征在于单晶结构分析,光谱方法(EDXS,FTIR,拉曼,UV / Vis,Mössbauer),热重分析法和密度泛函理论方法。
更新日期:2020-01-23
中文翻译:

[SnI8 {Fe(CO)4} 4] 2+:具有易碎羰基夹的高度配位的Sn + II I8亚基。
[SnI8 {Fe(CO)4} 4] [Al2 Cl7] 2包含[SnI8 {Fe(CO)4} 4] 2+阳离子,该阳离子具有空前的高度配位的,二配位的SnI8棱镜。考虑到与最大量的稳定卤化物的八重配合,更令人惊讶的是,这种SnI8排列仅以易碎的Fe(CO)4基团以夹子状的方式包围。尽管在[SnI8 {Fe(CO)4} 4] 2+中离子键占主导地位,但I-⋅⋅⋅I-距离明显缩短(低至371 pm),并且显着小于范德华距离( 420 pm)。标题化合物的特征在于单晶结构分析,光谱方法(EDXS,FTIR,拉曼,UV / Vis,Mössbauer),热重分析法和密度泛函理论方法。






































 京公网安备 11010802027423号
京公网安备 11010802027423号