当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal/LiF/Li2O Nanocomposite for Battery Cathode Prelithiation: Trade-off between Capacity and Stability.
Nano Letters ( IF 9.6 ) Pub Date : 2019-12-04 , DOI: 10.1021/acs.nanolett.9b04278 Junmou Du 1 , Wenyu Wang 1 , Alex Yong Sheng Eng 2 , Xiaoxiao Liu 1 , Mintao Wan 1 , Zhi Wei Seh 2 , Yongming Sun 1
Nano Letters ( IF 9.6 ) Pub Date : 2019-12-04 , DOI: 10.1021/acs.nanolett.9b04278 Junmou Du 1 , Wenyu Wang 1 , Alex Yong Sheng Eng 2 , Xiaoxiao Liu 1 , Mintao Wan 1 , Zhi Wei Seh 2 , Yongming Sun 1
Affiliation
|
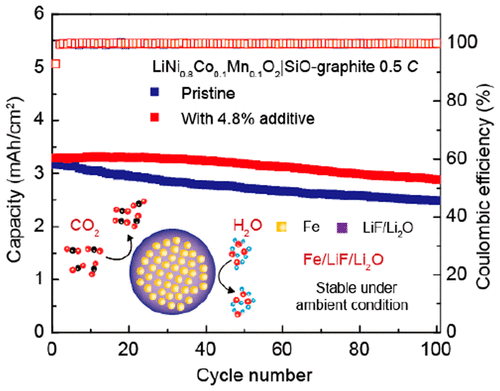
Lithium-ion batteries (LIBs) are currently dominating the portable electronics market and supplying power for electric vehicles and grid-level storage. However, lithium loss in the formation cycle at the anode side reduces the energy density of state-of-the-art LIBs with carbon anode materials. This situation will be even more severe for future LIBs using high-capacity Si-based anode materials. In this study, a transition metal-based nanocomposite with built-in lithium source was synthesized, featuring Fe nanodomains with a size of ∼5 nm uniformly dispersed in a hybrid Li2O and LiF matrix with intimate contact between them. The Fe/LiF/Li2O nanocomposite released a high Li-ion capacity of 550 mA h/g based on a multielectron inverse conversion reaction during the first-cycle charge process and exhibited better ambient stability than the counterpart with a pure Li2O matrix and also a lower lithium-extraction voltage and faster reaction kinetics than the counterpart with a pure LiF matrix. Serving as an additive to various cathodes (e.g., LiCoO2, LiFePO4, and LiNi1-x-yCoxMnyO2), the Fe/LiF/Li2O nanocomposite showed excellent lithium compensation effect. Using 4.8 wt % Fe/LiF/Li2O additive based on the total mass of the electrodes, a LiNi0.8Co0.1Mn0.1O2|SiO-graphite full cell with a high cathode mass loading of 20 mg/cm2 exhibited a high reversible capacity of 2.9 mA h/cm2 at 0.5 C after 100 cycles which is a 15% increase in comparison to the counterpart without the prelithiation additive. After the Fe/LiF/Li2O nanocomposite was immersed into the electrolyte and rested for 72 h, the content of iron metal in the electrolyte was negligible, indicating that this prelithiation additive was stable in the electrolyte and would not cause any side reactions, such as the shuttle of iron ions during cycling. The high "donor" Li-ion capacity, good ambient stability, and its compatibility with existing cathode materials and battery fabrication processes make the Fe/LiF/Li2O nanocomposite a promising cathode prelithiation additive to offset the initial lithium loss and improve the energy density of LIBs.
中文翻译:

用于电池阴极预锂化的金属/ LiF / Li2O纳米复合材料:容量和稳定性之间的权衡。
锂离子电池(LIB)目前在便携式电子市场中占主导地位,并为电动汽车和电网级存储供电。然而,在阳极侧的形成循环中的锂损失降低了具有碳阳极材料的最新型LIB的能量密度。对于使用高容量硅基负极材料的未来LIB,这种情况将更加严峻。在这项研究中,合成了具有内置锂源的过渡金属基纳米复合材料,其特征是Fe纳米畴尺寸约为5 nm,均匀分布在混合的Li2O和LiF基质中,并且彼此紧密接触。Fe / LiF / Li2O纳米复合材料在第一周期充电过程中通过多电子逆转换反应释放了550 mA h / g的高锂离子容量,并且与纯Li2O基质和锂离子电池相比,具有更好的环境稳定性。与纯LiF基质相比,具有更低的锂提取电压和更快的反应动力学。Fe / LiF / Li2O纳米复合材料用作各种阴极(例如LiCoO2,LiFePO4和LiNi1-x-yCoxMnyO2)的添加剂,具有出色的锂补偿效果。使用基于电极总质量的4.8 wt%Fe / LiF / Li2O添加剂,具有20 mg / cm2的高阴极质量负载的LiNi0.8Co0.1Mn0.1O2 | SiO-石墨全电池显示出高的可逆容量0时为2.9 mA h / cm2。100次循环后温度为5 C,与没有预锂化添加剂的情况相比增加了15%。将Fe / LiF / Li2O纳米复合材料浸入电解质中并静置72小时后,电解质中的铁金属含量可忽略不计,这表明该预锂化添加剂在电解质中稳定且不会引起任何副反应,例如循环过程中铁离子的穿梭。高的“施主”锂离子容量,良好的环境稳定性以及与现有阴极材料和电池制造工艺的相容性,使Fe / LiF / Li2O纳米复合材料成为有前途的阴极预锂化添加剂,可抵消初始锂损失并提高能量密度。 LIB。电解质中铁金属的含量可以忽略不计,表明该预锂化添加剂在电解质中稳定,不会引起任何副反应,如循环过程中铁离子的穿梭。高的“施主”锂离子容量,良好的环境稳定性以及与现有阴极材料和电池制造工艺的相容性,使Fe / LiF / Li2O纳米复合材料成为有前途的阴极预锂化添加剂,可抵消初始锂损失并提高能量密度。 LIB。电解质中铁金属的含量可以忽略不计,表明该预锂化添加剂在电解质中稳定,不会引起任何副反应,如循环过程中铁离子的穿梭。高的“施主”锂离子容量,良好的环境稳定性以及与现有阴极材料和电池制造工艺的相容性,使Fe / LiF / Li2O纳米复合材料成为有前途的阴极预锂化添加剂,可抵消初始锂损失并提高能量密度。 LIB。
更新日期:2019-12-04
中文翻译:

用于电池阴极预锂化的金属/ LiF / Li2O纳米复合材料:容量和稳定性之间的权衡。
锂离子电池(LIB)目前在便携式电子市场中占主导地位,并为电动汽车和电网级存储供电。然而,在阳极侧的形成循环中的锂损失降低了具有碳阳极材料的最新型LIB的能量密度。对于使用高容量硅基负极材料的未来LIB,这种情况将更加严峻。在这项研究中,合成了具有内置锂源的过渡金属基纳米复合材料,其特征是Fe纳米畴尺寸约为5 nm,均匀分布在混合的Li2O和LiF基质中,并且彼此紧密接触。Fe / LiF / Li2O纳米复合材料在第一周期充电过程中通过多电子逆转换反应释放了550 mA h / g的高锂离子容量,并且与纯Li2O基质和锂离子电池相比,具有更好的环境稳定性。与纯LiF基质相比,具有更低的锂提取电压和更快的反应动力学。Fe / LiF / Li2O纳米复合材料用作各种阴极(例如LiCoO2,LiFePO4和LiNi1-x-yCoxMnyO2)的添加剂,具有出色的锂补偿效果。使用基于电极总质量的4.8 wt%Fe / LiF / Li2O添加剂,具有20 mg / cm2的高阴极质量负载的LiNi0.8Co0.1Mn0.1O2 | SiO-石墨全电池显示出高的可逆容量0时为2.9 mA h / cm2。100次循环后温度为5 C,与没有预锂化添加剂的情况相比增加了15%。将Fe / LiF / Li2O纳米复合材料浸入电解质中并静置72小时后,电解质中的铁金属含量可忽略不计,这表明该预锂化添加剂在电解质中稳定且不会引起任何副反应,例如循环过程中铁离子的穿梭。高的“施主”锂离子容量,良好的环境稳定性以及与现有阴极材料和电池制造工艺的相容性,使Fe / LiF / Li2O纳米复合材料成为有前途的阴极预锂化添加剂,可抵消初始锂损失并提高能量密度。 LIB。电解质中铁金属的含量可以忽略不计,表明该预锂化添加剂在电解质中稳定,不会引起任何副反应,如循环过程中铁离子的穿梭。高的“施主”锂离子容量,良好的环境稳定性以及与现有阴极材料和电池制造工艺的相容性,使Fe / LiF / Li2O纳米复合材料成为有前途的阴极预锂化添加剂,可抵消初始锂损失并提高能量密度。 LIB。电解质中铁金属的含量可以忽略不计,表明该预锂化添加剂在电解质中稳定,不会引起任何副反应,如循环过程中铁离子的穿梭。高的“施主”锂离子容量,良好的环境稳定性以及与现有阴极材料和电池制造工艺的相容性,使Fe / LiF / Li2O纳米复合材料成为有前途的阴极预锂化添加剂,可抵消初始锂损失并提高能量密度。 LIB。































 京公网安备 11010802027423号
京公网安备 11010802027423号