当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Difunctionalization of Cyclopropyl Amines with N-Iodosuccinimide (NIS) or in Situ Formed Cyanogen Iodide (ICN).
Organic Letters ( IF 4.9 ) Pub Date : 2019-11-27 , DOI: 10.1021/acs.orglett.9b03922 Qile Wang 1 , Nan Zheng 1
Organic Letters ( IF 4.9 ) Pub Date : 2019-11-27 , DOI: 10.1021/acs.orglett.9b03922 Qile Wang 1 , Nan Zheng 1
Affiliation
|
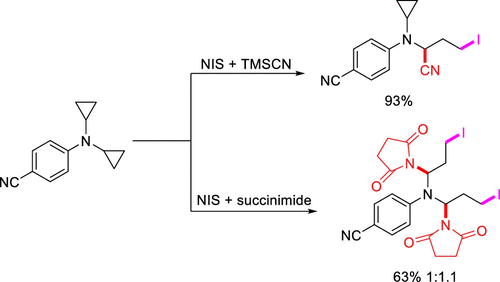
We report herein a 1,3-difunctionalization of cyclopropylamines that serve as a π nucleophile in a two-electron (2e) SN2-like ring opening pathway. N-Iodosuccinimide (NIS) or in situ generated cyanogen iodide (ICN) is employed as electrophilic iodinating reagents in conjunction with TMSCN or succinimide to furnish multiple pairs of functional groups disposed in a 1,3-manner. This 2e ring opening manifold overcomes the constraint of our previously reported 1e protocol as demonstrated by successful activation of monocyclic tertiary cyclopropylamines.
中文翻译:

用N-碘代琥珀酰亚胺(NIS)或原位形成的氰化碘化物(ICN)对环丙基胺进行双官能化。
我们在这里报道了在两个电子(2e)SN2样开环途径中充当π亲核体的环丙胺的1,3-双官能化。N-碘代琥珀酰亚胺(NIS)或原位生成的碘化氰(ICN)与TMSCN或琥珀酰亚胺一起用作亲电子碘化试剂,以提供多对以1,3-方式布置的官能团。该2e开环歧管克服了我们先前报道的1e方案的局限性,如成功激活单环叔环丙胺所证明的。
更新日期:2019-11-28
中文翻译:

用N-碘代琥珀酰亚胺(NIS)或原位形成的氰化碘化物(ICN)对环丙基胺进行双官能化。
我们在这里报道了在两个电子(2e)SN2样开环途径中充当π亲核体的环丙胺的1,3-双官能化。N-碘代琥珀酰亚胺(NIS)或原位生成的碘化氰(ICN)与TMSCN或琥珀酰亚胺一起用作亲电子碘化试剂,以提供多对以1,3-方式布置的官能团。该2e开环歧管克服了我们先前报道的1e方案的局限性,如成功激活单环叔环丙胺所证明的。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号