当前位置:
X-MOL 学术
›
Anal. Methods
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In situ hemolysis in a three-dimensional paper-based device for quantification of intraerythrocytic analytes
Analytical Methods ( IF 2.7 ) Pub Date : 2019-11-27 , DOI: 10.1039/c9ay02292a Keith R. Baillargeon 1, 2, 3, 4, 5 , Jordan R. Bricknell 1, 2, 3, 4, 5 , Charles R. Mace 1, 2, 3, 4, 5
Analytical Methods ( IF 2.7 ) Pub Date : 2019-11-27 , DOI: 10.1039/c9ay02292a Keith R. Baillargeon 1, 2, 3, 4, 5 , Jordan R. Bricknell 1, 2, 3, 4, 5 , Charles R. Mace 1, 2, 3, 4, 5
Affiliation
|
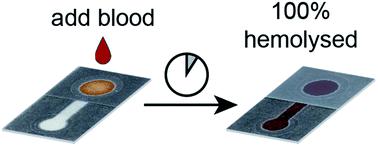
Blood-based diagnostics require various forms of sample preparation depending on the analyte of interest, which can include plasma separation and cellular lysis. Specifically, assays that require the release of intraerythrocytic analytes (e.g., detection of malaria antigens, dehydrogenases, and hemoglobin) require the rupture of red blood cells prior to analysis. Associated handling steps and additional fluid manipulation complicates the user-experience by adding time, potential for contamination error, and reagent waste. In this work, we demonstrate an in situ chemical hemolysis treatment coupled with a paper-based device for the quantification of liberated hemoglobin without using a hemolytic buffer. In contrast to traditional hemolytic methods that use a buffered solution of saponin, a surfactant, we dried saponin within our device to lyse red blood cells without diluting the sample. The optimal treatment condition for hemolysis of blood samples with hematocrit values ranging from 20–50% was 10.6 μg saponin per cm2. Establishing a relationship between saponin and zone area potentially allows this in situ hemolysis treatment to be translated to other paper-based devices with different geometries. For samples with hematocrit values below 40%, we achieved quantitative hemolysis. Samples with higher hematocrits (e.g., 40–50%) experienced a lesser extent of hemolysis (80–85%), which we attribute to the increased number of red blood cells present in samples with elevated hematocrits. The in situ chemical hemolysis treatment described here could potentially be integrated with a multiplexed paper-based microfluidic device to permit multiple sample preparation techniques on a single sample of blood without additional off-chip user steps.
中文翻译:

在三维纸基设备中进行原位溶血以定量红细胞内分析物
基于血液的诊断需要根据目标分析物的不同形式的样品制备,其中包括血浆分离和细胞裂解。具体而言,需要释放红细胞内分析物的测定(例如,检测疟疾抗原,脱氢酶和血红蛋白)需要在分析之前使红细胞破裂。相关的处理步骤和额外的流体操作会增加时间,污染错误的可能性和试剂浪费,从而使用户的体验复杂化。在这项工作中,我们将现场演示化学溶血治疗,结合基于纸的设备,可定量释放的血红蛋白,而无需使用溶血缓冲液。与使用表面活性剂皂苷缓冲溶液的传统溶血方法相反,我们在设备中干燥了皂苷以溶解红细胞而不稀释样品。血细胞比容值范围为20–50%的血液样品溶血的最佳治疗条件是每cm 2含10.6μg皂苷。在皂苷和区域面积之间建立关系可能使这种原位溶血治疗转化为其他具有不同几何形状的纸基设备。对于血细胞比容值低于40%的样品,我们实现了定量溶血。血细胞比容较高的样品(例如,40–50%)的溶血程度较小(80–85%),这归因于血细胞比容升高的样品中存在的红细胞数量增加。该原位这里所描述的化学溶血处理可以潜在地与多路复用纸基微流体装置集成,以允许对血液单个样品而无需额外的芯片外的用户步骤的多个样品制备技术。
更新日期:2019-11-27
中文翻译:

在三维纸基设备中进行原位溶血以定量红细胞内分析物
基于血液的诊断需要根据目标分析物的不同形式的样品制备,其中包括血浆分离和细胞裂解。具体而言,需要释放红细胞内分析物的测定(例如,检测疟疾抗原,脱氢酶和血红蛋白)需要在分析之前使红细胞破裂。相关的处理步骤和额外的流体操作会增加时间,污染错误的可能性和试剂浪费,从而使用户的体验复杂化。在这项工作中,我们将现场演示化学溶血治疗,结合基于纸的设备,可定量释放的血红蛋白,而无需使用溶血缓冲液。与使用表面活性剂皂苷缓冲溶液的传统溶血方法相反,我们在设备中干燥了皂苷以溶解红细胞而不稀释样品。血细胞比容值范围为20–50%的血液样品溶血的最佳治疗条件是每cm 2含10.6μg皂苷。在皂苷和区域面积之间建立关系可能使这种原位溶血治疗转化为其他具有不同几何形状的纸基设备。对于血细胞比容值低于40%的样品,我们实现了定量溶血。血细胞比容较高的样品(例如,40–50%)的溶血程度较小(80–85%),这归因于血细胞比容升高的样品中存在的红细胞数量增加。该原位这里所描述的化学溶血处理可以潜在地与多路复用纸基微流体装置集成,以允许对血液单个样品而无需额外的芯片外的用户步骤的多个样品制备技术。











































 京公网安备 11010802027423号
京公网安备 11010802027423号