Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal-free synthesis of 1,N6-ethenoadenines from N6-propargyl-adenines via NIS mediated radical cascade reaction
RSC Advances ( IF 3.9 ) Pub Date : 2019-11-26 , DOI: 10.1039/c9ra09198j Ruchun Yang 1, 2 , Si Deng 2 , Xiang-You Dong 2 , Xianrong Song 2 , Hu Cai 1 , Jiang Bai 2 , Qiang Xiao 2
RSC Advances ( IF 3.9 ) Pub Date : 2019-11-26 , DOI: 10.1039/c9ra09198j Ruchun Yang 1, 2 , Si Deng 2 , Xiang-You Dong 2 , Xianrong Song 2 , Hu Cai 1 , Jiang Bai 2 , Qiang Xiao 2
Affiliation
|
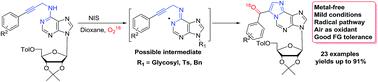
In the present paper, an efficient approach for the construction of 1,N6-ethenoadenines from conveniently prepared N6-propargyl-adenines is developed. This reaction merges N-iodosuccinimide radical initiation and aerobic aminooxygenation in dioxane. This mild, 5-exo-dig, and metal-free cascade reaction could be applied to a wide substrate scope to provide 1,N6-ethenoadenines in moderate to good yields. The reaction mechanism was proposed and tested using radical inhibitor (butylated hydroxytoluene) and isotopic labelling (18O2) experiments.
中文翻译:

N6-炔丙基-腺嘌呤通过 NIS 介导的自由基级联反应无金属合成 1,N6-乙烯腺嘌呤
在本文中,开发了一种从方便制备的N 6 -炔丙基-腺嘌呤构建 1, N 6 -亚乙基腺嘌呤的有效方法。该反应合并了N-碘代琥珀酰亚胺自由基引发和二恶烷中的需氧氨基氧化。这种温和的5-exo-dig和无金属级联反应可应用于广泛的底物范围,以中等至良好的产率提供 1, N 6 -亚乙基腺嘌呤。使用自由基抑制剂(丁基化羟基甲苯)和同位素标记(18 O 2)实验提出并测试了反应机理。
更新日期:2019-11-26
中文翻译:

N6-炔丙基-腺嘌呤通过 NIS 介导的自由基级联反应无金属合成 1,N6-乙烯腺嘌呤
在本文中,开发了一种从方便制备的N 6 -炔丙基-腺嘌呤构建 1, N 6 -亚乙基腺嘌呤的有效方法。该反应合并了N-碘代琥珀酰亚胺自由基引发和二恶烷中的需氧氨基氧化。这种温和的5-exo-dig和无金属级联反应可应用于广泛的底物范围,以中等至良好的产率提供 1, N 6 -亚乙基腺嘌呤。使用自由基抑制剂(丁基化羟基甲苯)和同位素标记(18 O 2)实验提出并测试了反应机理。































 京公网安备 11010802027423号
京公网安备 11010802027423号