当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The in vivo ISGylome links ISG15 to metabolic pathways and autophagy upon Listeria monocytogenes infection.
Nature Communications ( IF 14.7 ) Pub Date : 2019-11-26 , DOI: 10.1038/s41467-019-13393-x Yifeng Zhang 1 , Fabien Thery 2, 3 , Nicholas C Wu 4 , Emma K Luhmann 1 , Olivier Dussurget 5, 6, 7 , Mariko Foecke 5, 6, 7 , Clara Bredow 8 , Daniel Jiménez-Fernández 9 , Kevin Leandro 2, 3 , Antje Beling 8, 10 , Klaus-Peter Knobeloch 9 , Francis Impens 2, 3, 11 , Pascale Cossart 5, 6, 7 , Lilliana Radoshevich 1
Nature Communications ( IF 14.7 ) Pub Date : 2019-11-26 , DOI: 10.1038/s41467-019-13393-x Yifeng Zhang 1 , Fabien Thery 2, 3 , Nicholas C Wu 4 , Emma K Luhmann 1 , Olivier Dussurget 5, 6, 7 , Mariko Foecke 5, 6, 7 , Clara Bredow 8 , Daniel Jiménez-Fernández 9 , Kevin Leandro 2, 3 , Antje Beling 8, 10 , Klaus-Peter Knobeloch 9 , Francis Impens 2, 3, 11 , Pascale Cossart 5, 6, 7 , Lilliana Radoshevich 1
Affiliation
|
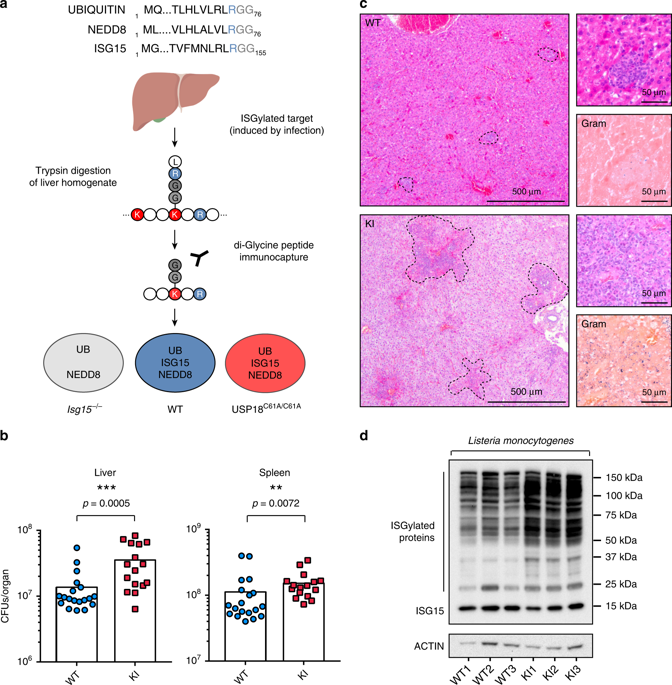
ISG15 is an interferon-stimulated, ubiquitin-like protein, with anti-viral and anti-bacterial activity. Here, we map the endogenous in vivo ISGylome in the liver following Listeria monocytogenes infection by combining murine models of reduced or enhanced ISGylation with quantitative proteomics. Our method identifies 930 ISG15 sites in 434 proteins and also detects changes in the host ubiquitylome. The ISGylated targets are enriched in proteins which alter cellular metabolic processes, including upstream modulators of the catabolic and antibacterial pathway of autophagy. Computational analysis of substrate structures reveals that a number of ISG15 modifications occur at catalytic sites or dimerization interfaces of enzymes. Finally, we demonstrate that animals and cells with enhanced ISGylation have increased basal and infection-induced autophagy through the modification of mTOR, WIPI2, AMBRA1, and RAB7. Taken together, these findings ascribe a role of ISGylation to temporally reprogram organismal metabolism following infection through direct modification of a subset of enzymes in the liver.
中文翻译:

体内 ISGylome 将 ISG15 与单核细胞增生李斯特菌感染时的代谢途径和自噬联系起来。
ISG15 是一种干扰素刺激的泛素样蛋白,具有抗病毒和抗菌活性。在这里,我们通过将 ISGylation 减少或增强的小鼠模型与定量蛋白质组学相结合,绘制了单核细胞增多性李斯特菌感染后肝脏中的内源性体内 ISGylome。我们的方法鉴定了 434 个蛋白质中的 930 个 ISG15 位点,还检测了宿主泛素组的变化。 ISGylated 靶标富含改变细胞代谢过程的蛋白质,包括自噬分解代谢和抗菌途径的上游调节剂。底物结构的计算分析表明,许多 ISG15 修饰发生在酶的催化位点或二聚化界面。最后,我们证明 ISGylation 增强的动物和细胞通过 mTOR、WIPI2、AMBRA1 和 RAB7 的修饰增加了基础自噬和感染诱导的自噬。总而言之,这些发现归因于 ISGylation 通过直接修饰肝脏中的一部分酶来暂时重新编程感染后的有机体代谢。
更新日期:2019-11-27
中文翻译:

体内 ISGylome 将 ISG15 与单核细胞增生李斯特菌感染时的代谢途径和自噬联系起来。
ISG15 是一种干扰素刺激的泛素样蛋白,具有抗病毒和抗菌活性。在这里,我们通过将 ISGylation 减少或增强的小鼠模型与定量蛋白质组学相结合,绘制了单核细胞增多性李斯特菌感染后肝脏中的内源性体内 ISGylome。我们的方法鉴定了 434 个蛋白质中的 930 个 ISG15 位点,还检测了宿主泛素组的变化。 ISGylated 靶标富含改变细胞代谢过程的蛋白质,包括自噬分解代谢和抗菌途径的上游调节剂。底物结构的计算分析表明,许多 ISG15 修饰发生在酶的催化位点或二聚化界面。最后,我们证明 ISGylation 增强的动物和细胞通过 mTOR、WIPI2、AMBRA1 和 RAB7 的修饰增加了基础自噬和感染诱导的自噬。总而言之,这些发现归因于 ISGylation 通过直接修饰肝脏中的一部分酶来暂时重新编程感染后的有机体代谢。































 京公网安备 11010802027423号
京公网安备 11010802027423号