当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Zinc anode-compatible in-situ solid electrolyte interphase via cation solvation modulation.
Nature Communications ( IF 14.7 ) Pub Date : 2019-11-26 , DOI: 10.1038/s41467-019-13436-3
Huayu Qiu 1, 2 , Xiaofan Du 1 , Jingwen Zhao 1 , Yantao Wang 1 , Jiangwei Ju 1 , Zheng Chen 1 , Zhenglin Hu 1 , Dongpeng Yan 3 , Xinhong Zhou 2 , Guanglei Cui 1
Nature Communications ( IF 14.7 ) Pub Date : 2019-11-26 , DOI: 10.1038/s41467-019-13436-3
Huayu Qiu 1, 2 , Xiaofan Du 1 , Jingwen Zhao 1 , Yantao Wang 1 , Jiangwei Ju 1 , Zheng Chen 1 , Zhenglin Hu 1 , Dongpeng Yan 3 , Xinhong Zhou 2 , Guanglei Cui 1
Affiliation
|
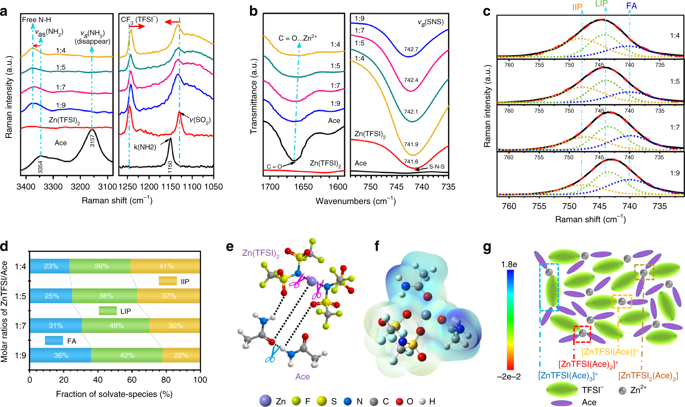
The surface chemistry of solid electrolyte interphase is one of the critical factors that govern the cycling life of rechargeable batteries. However, this chemistry is less explored for zinc anodes, owing to their relatively high redox potential and limited choices in electrolyte. Here, we report the observation of a zinc fluoride-rich organic/inorganic hybrid solid electrolyte interphase on zinc anode, based on an acetamide-Zn(TFSI)2 eutectic electrolyte. A combination of experimental and modeling investigations reveals that the presence of anion-complexing zinc species with markedly lowered decomposition energies contributes to the in situ formation of an interphase. The as-protected anode enables reversible (~100% Coulombic efficiency) and dendrite-free zinc plating/stripping even at high areal capacities (>2.5 mAh cm‒2), endowed by the fast ion migration coupled with high mechanical strength of the protective interphase. With this interphasial design the assembled zinc batteries exhibit excellent cycling stability with negligible capacity loss at both low and high rates.
中文翻译:

通过阳离子溶剂化调节可与锌阳极相容的原位固体电解质中间相。
固体电解质中间相的表面化学性质是控制可充电电池循环寿命的关键因素之一。然而,由于锌阳极具有较高的氧化还原电势和在电解质中的有限选择,因此对锌阳极的化学研究较少。在这里,我们报告了基于乙酰胺-Zn(TFSI)2低共熔电解质的锌阳极上富含氟化锌的有机/无机混合固体电解质中间相的观察结果。实验研究和模型研究的结合表明,具有明显降低的分解能的阴离子络合锌物质的存在有助于中间相的原位形成。处于保护状态的阳极即使在高面积容量(> 2.5 mAh cm‒2)时也可实现可逆的(〜100%库仑效率)和无枝晶的镀锌/剥离,离子迁移迅速,加上保护相的机械强度高。通过这种相间设计,组装好的锌电池在低速和高倍率下均具有出色的循环稳定性和可忽略不计的容量损失。
更新日期:2019-11-27
中文翻译:

通过阳离子溶剂化调节可与锌阳极相容的原位固体电解质中间相。
固体电解质中间相的表面化学性质是控制可充电电池循环寿命的关键因素之一。然而,由于锌阳极具有较高的氧化还原电势和在电解质中的有限选择,因此对锌阳极的化学研究较少。在这里,我们报告了基于乙酰胺-Zn(TFSI)2低共熔电解质的锌阳极上富含氟化锌的有机/无机混合固体电解质中间相的观察结果。实验研究和模型研究的结合表明,具有明显降低的分解能的阴离子络合锌物质的存在有助于中间相的原位形成。处于保护状态的阳极即使在高面积容量(> 2.5 mAh cm‒2)时也可实现可逆的(〜100%库仑效率)和无枝晶的镀锌/剥离,离子迁移迅速,加上保护相的机械强度高。通过这种相间设计,组装好的锌电池在低速和高倍率下均具有出色的循环稳定性和可忽略不计的容量损失。

































 京公网安备 11010802027423号
京公网安备 11010802027423号