当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Volatile Etch Species Produced during Thermal Al2O3 Atomic Layer Etching
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-12-17 , DOI: 10.1021/acs.jpcc.9b06104
Joel W. Clancey 1 , Andrew S. Cavanagh 1 , James E. T. Smith 1 , Sandeep Sharma 1 , Steven M. George 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-12-17 , DOI: 10.1021/acs.jpcc.9b06104
Joel W. Clancey 1 , Andrew S. Cavanagh 1 , James E. T. Smith 1 , Sandeep Sharma 1 , Steven M. George 1
Affiliation
|
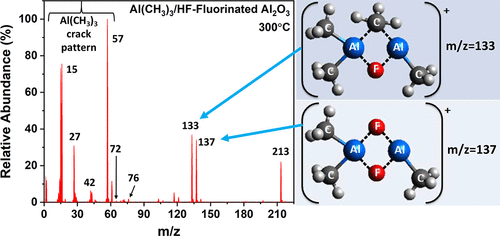
The thermal atomic layer etching (ALE) of Al2O3 can be achieved using sequential fluorination and ligand-exchange reactions. Although previous investigations have characterized the etch rates and surface chemistry, no reports have identified the volatile etch products. This study explored the volatile etch species during thermal Al2O3 ALE at 300 °C using quadrupole mass spectrometry (QMS). HF was the fluorination reactant; Al(CH3)3 (trimethylaluminum (TMA)) and AlCl(CH3)2 (dimethylaluminum chloride, (DMAC)) were the metal precursors for ligand exchange. When TMA was used as the metal precursor after the fluorination of Al2O3 powder, the QMS measurements revealed that the main ion species were consistent with dimers of AlF(CH3)2 (dimethylaluminum fluoride (DMAF)) with itself (DMAF + DMAF) or with TMA (DMAF + TMA). These ion species were observed after loss of a methyl group as Al2F2(CH3)3+ at m/z = 137 and Al2F(CH3)4+ at m/z = 133, respectively. In addition, an ion species consistent with a trimer was also observed as Al3F3(CH3)5+ at m/z = 213. Very similar results were observed for TMA exposures on AlF3 powder. Comparable results were also obtained using DMAC as the metal precursor for ligand exchange. In contrast, SiCl4 and TiCl4 are not successful metal precursors because they do not lead to thermal Al2O3 ALE at 300 °C. QMS measurements revealed no Al-containing etch species after SiCl4 and TiCl4 exposures on AlF3 powder. However, SiFxCly+ and TiFxCly+ species were observed which suggested that ligand-exchange reactions can occur without the release of Al-containing etch species. Density functional theory (DFT) and coupled cluster singles, doubles, and perturbative triples (CCSD(T)) calculations were performed to support the preference for dimer products. The theoretical results confirmed the stability of the dimer products and showed that dimers with two Al–F–Al bridging bonds are the most stable and dimers with two Al–CH3–Al bridging bonds are the least stable. In addition, the calculations suggested that dimers with terminal CH3 ligands are most able to desorb from the surface because these dimers need to break weak Al–CH3–Al bridging bonds. Transmission electron microscopy (TEM) studies confirmed the thermal Al2O3 ALE of Al2O3 films on W powders. The TEM images revealed that the etch process was uniform and conformal after various numbers of thermal Al2O3 ALE cycles using HF and TMA as the reactants.
中文翻译:

Al 2 O 3原子层热刻蚀过程中产生的挥发性刻蚀物种
Al 2 O 3的热原子层蚀刻(ALE)可以使用顺序氟化和配体交换反应来实现。尽管先前的研究已经刻蚀了腐蚀速率和表面化学特性,但尚无任何报告确定挥发性腐蚀产物。这项研究使用四极杆质谱(QMS)探索了在300°C的热Al 2 O 3 ALE期间的挥发性蚀刻物质。HF是氟化反应物。Al(CH 3)3(三甲基铝(TMA))和AlCl(CH 3)2(二甲基氯化铝,(DMAC))是用于配体交换的金属前体。铝氟化后使用TMA作为金属前体时在2 O 3粉末中,QMS测量显示主要离子种类与AlF(CH 3)2(氟化二甲基铝(DMAF))本身(DMAF + DMAF)或TMA(DMAF + TMA)的二聚体一致。甲基的损失以Al后观察到这些离子物质2 ˚F 2(CH 3)3 +,在米/ Ž = 137和Al 2 F(CH 3)4 +以米/ Ž = 133,分别。此外,三聚体相一致的离子种,也观察到以Al 3 ˚F3(CH 3)5 +的m / z =213。在AlF 3粉末上的TMA暴露观察到非常相似的结果。使用DMAC作为配体交换的金属前体,也获得了可比的结果。相反,SiCl 4和TiCl 4并不是成功的金属前体,因为它们不会导致300°C的Al 2 O 3 ALE热。QMS测量显示在AlF 3粉末上暴露了SiCl 4和TiCl 4之后,没有含铝的蚀刻物质。但是,SiF x Cl y +和TiF观察到x Cl y +物种,这表明可以发生配体交换反应而不会释放含铝的蚀刻物种。进行密度泛函理论(DFT)和耦合的簇单,双和微扰三元组(CCSD(T))计算,以支持对二聚体产物的偏爱。理论结果证实了二聚体产物的稳定性,表明具有两个Al–F–Al桥键的二聚体最稳定,具有两个Al–CH 3 –Al桥键的二聚体最不稳定。此外,计算结果表明,带有末端CH 3配体的二聚体最能从表面脱附,因为这些二聚体需要破坏弱的Al–CH 3。–铝桥联债券。透射电子显微镜(TEM)研究证实了W粉上的Al 2 O 3膜具有热Al 2 O 3 ALE 。TEM图像显示,使用HF和TMA作为反应物,经过不同数量的Al 2 O 3 ALE热循环后,蚀刻过程是均匀且共形的。
更新日期:2019-12-18
中文翻译:

Al 2 O 3原子层热刻蚀过程中产生的挥发性刻蚀物种
Al 2 O 3的热原子层蚀刻(ALE)可以使用顺序氟化和配体交换反应来实现。尽管先前的研究已经刻蚀了腐蚀速率和表面化学特性,但尚无任何报告确定挥发性腐蚀产物。这项研究使用四极杆质谱(QMS)探索了在300°C的热Al 2 O 3 ALE期间的挥发性蚀刻物质。HF是氟化反应物。Al(CH 3)3(三甲基铝(TMA))和AlCl(CH 3)2(二甲基氯化铝,(DMAC))是用于配体交换的金属前体。铝氟化后使用TMA作为金属前体时在2 O 3粉末中,QMS测量显示主要离子种类与AlF(CH 3)2(氟化二甲基铝(DMAF))本身(DMAF + DMAF)或TMA(DMAF + TMA)的二聚体一致。甲基的损失以Al后观察到这些离子物质2 ˚F 2(CH 3)3 +,在米/ Ž = 137和Al 2 F(CH 3)4 +以米/ Ž = 133,分别。此外,三聚体相一致的离子种,也观察到以Al 3 ˚F3(CH 3)5 +的m / z =213。在AlF 3粉末上的TMA暴露观察到非常相似的结果。使用DMAC作为配体交换的金属前体,也获得了可比的结果。相反,SiCl 4和TiCl 4并不是成功的金属前体,因为它们不会导致300°C的Al 2 O 3 ALE热。QMS测量显示在AlF 3粉末上暴露了SiCl 4和TiCl 4之后,没有含铝的蚀刻物质。但是,SiF x Cl y +和TiF观察到x Cl y +物种,这表明可以发生配体交换反应而不会释放含铝的蚀刻物种。进行密度泛函理论(DFT)和耦合的簇单,双和微扰三元组(CCSD(T))计算,以支持对二聚体产物的偏爱。理论结果证实了二聚体产物的稳定性,表明具有两个Al–F–Al桥键的二聚体最稳定,具有两个Al–CH 3 –Al桥键的二聚体最不稳定。此外,计算结果表明,带有末端CH 3配体的二聚体最能从表面脱附,因为这些二聚体需要破坏弱的Al–CH 3。–铝桥联债券。透射电子显微镜(TEM)研究证实了W粉上的Al 2 O 3膜具有热Al 2 O 3 ALE 。TEM图像显示,使用HF和TMA作为反应物,经过不同数量的Al 2 O 3 ALE热循环后,蚀刻过程是均匀且共形的。


































 京公网安备 11010802027423号
京公网安备 11010802027423号