当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical CO2 Reduction Reaction on [email protected](211) Bimetallic Single-Atom Surface Alloys: Mechanism, Kinetics, and Catalyst Screening
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2019-12-06 , DOI: 10.1021/acssuschemeng.9b05183 Yonghao Feng 1 , Wei An 1 , Zeming Wang 1 , Yuanqiang Wang 1 , Yong Men 1 , Yuanyuan Du 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2019-12-06 , DOI: 10.1021/acssuschemeng.9b05183 Yonghao Feng 1 , Wei An 1 , Zeming Wang 1 , Yuanqiang Wang 1 , Yong Men 1 , Yuanyuan Du 1
Affiliation
|
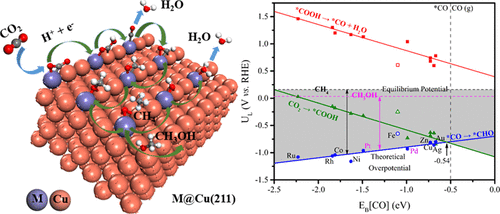
Copper is a well-known metal for catalyzing the electrochemical CO2 reduction reaction (CO2 RR) toward valuable hydrocarbons and alcohols. Here, using a combined density functional theory and microkinetic modeling approach, we systematically investigated 11 bimetallic [email protected](211) single-atom stepped surface alloys for their CO2 RR activity. It is revealed that the stepped M edge is most likely to be the active site for CO2 RR. The primary reaction pathway is identified as *COOH → *CO → *CHO with the potential-determining step of *CO + H+ + e– → *CHO, leading to either CH4 or CH3OH formation at more negative potential. Especially, [email protected](211) and [email protected](211) are both predicted to be most efficient in promoting CO2 RR toward CH4 owing to their breaking of the coupled scaling relations of key intermediates’ binding at the active site. Furthermore, the binding strength of *CO and *OH can be used as a good descriptor for differentiating various [email protected](211) for CO2 RR activity and selectivity, and specifically, the moderate oxophilic and carbophilic elements of M are preferred. Our study highlights the utmost importance of breaking the linear scaling relations of key intermediates’ binding at the active site for boosting CO2 RR performance.
中文翻译:

[电子邮件保护](211)双金属单原子表面合金上的电化学CO 2还原反应:机理,动力学和催化剂筛选
铜是用于催化电化学的CO 2还原反应(CO 2 RR)转化为有价值的烃和醇的众所周知的金属。在这里,使用密度泛函理论和微动力学建模方法相结合,我们系统地研究了11种双金属[受电子邮件保护](211)单原子阶梯式表面合金的CO 2 RR活性。揭示出阶梯状的M边缘最有可能是CO 2 RR的活性位点。主要反应途径为* COOH→* CO→* CHO,势能确定步骤为* CO + H + + e – →* CHO,导致CH 4或CH 3OH的形成更具负电势。特别是,[受电子邮件保护](211)和[受电子邮件保护](211)都被预测为最有效地促进CO 2 RR向CH 4的转化,因为它们破坏了关键中间体在活性位点结合的比例关系。 。此外,* CO和* OH的结合强度可以用作区分各种[电子邮件保护的](211)的CO 2 RR活性和选择性的良好描述,特别是,M的中等嗜氧和嗜热元素是优选的。我们的研究强调了打破关键中间体在活性位点结合的线性比例关系以提高CO 2 RR性能的最重要意义。
更新日期:2019-12-07
中文翻译:

[电子邮件保护](211)双金属单原子表面合金上的电化学CO 2还原反应:机理,动力学和催化剂筛选
铜是用于催化电化学的CO 2还原反应(CO 2 RR)转化为有价值的烃和醇的众所周知的金属。在这里,使用密度泛函理论和微动力学建模方法相结合,我们系统地研究了11种双金属[受电子邮件保护](211)单原子阶梯式表面合金的CO 2 RR活性。揭示出阶梯状的M边缘最有可能是CO 2 RR的活性位点。主要反应途径为* COOH→* CO→* CHO,势能确定步骤为* CO + H + + e – →* CHO,导致CH 4或CH 3OH的形成更具负电势。特别是,[受电子邮件保护](211)和[受电子邮件保护](211)都被预测为最有效地促进CO 2 RR向CH 4的转化,因为它们破坏了关键中间体在活性位点结合的比例关系。 。此外,* CO和* OH的结合强度可以用作区分各种[电子邮件保护的](211)的CO 2 RR活性和选择性的良好描述,特别是,M的中等嗜氧和嗜热元素是优选的。我们的研究强调了打破关键中间体在活性位点结合的线性比例关系以提高CO 2 RR性能的最重要意义。































 京公网安备 11010802027423号
京公网安备 11010802027423号