当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synergy between Cyclase-associated protein and Cofilin accelerates actin filament depolymerization by two orders of magnitude.
Nature Communications ( IF 14.7 ) Pub Date : 2019-11-22 , DOI: 10.1038/s41467-019-13268-1
Shashank Shekhar 1, 2, 3 , Johnson Chung 3 , Jane Kondev 2 , Jeff Gelles 3 , Bruce L Goode 1
Nature Communications ( IF 14.7 ) Pub Date : 2019-11-22 , DOI: 10.1038/s41467-019-13268-1
Shashank Shekhar 1, 2, 3 , Johnson Chung 3 , Jane Kondev 2 , Jeff Gelles 3 , Bruce L Goode 1
Affiliation
|
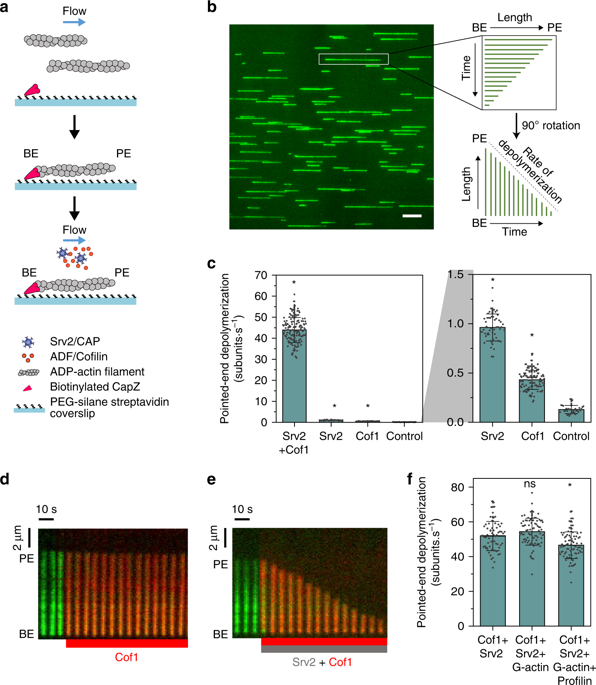
Cellular actin networks can be rapidly disassembled and remodeled in a few seconds, yet in vitro actin filaments depolymerize slowly over minutes. The cellular mechanisms enabling actin to depolymerize this fast have so far remained obscure. Using microfluidics-assisted TIRF, we show that Cyclase-associated protein (CAP) and Cofilin synergize to processively depolymerize actin filament pointed ends at a rate 330-fold faster than spontaneous depolymerization. Single molecule imaging further reveals that hexameric CAP molecules interact with the pointed ends of Cofilin-decorated filaments for several seconds at a time, removing approximately 100 actin subunits per binding event. These findings establish a paradigm, in which a filament end-binding protein and a side-binding protein work in concert to control actin dynamics, and help explain how rapid actin network depolymerization is achieved in cells.
中文翻译:

环化酶相关蛋白和 Cofilin 之间的协同作用可将肌动蛋白丝解聚加速两个数量级。
细胞肌动蛋白网络可以在几秒钟内快速分解和重塑,但体外肌动蛋白丝在几分钟内缓慢解聚。迄今为止,使肌动蛋白如此快速解聚的细胞机制仍不清楚。使用微流体辅助 TIRF,我们发现环化酶相关蛋白 (CAP) 和 Cofilin 协同作用,以比自发解聚快 330 倍的速度持续解聚肌动蛋白丝尖端。单分子成像进一步揭示,六聚体 CAP 分子与 Cofilin 修饰的细丝的尖端每次相互作用几秒钟,每次结合事件去除大约 100 个肌动蛋白亚基。这些发现建立了一个范例,其中丝末端结合蛋白和侧结合蛋白协同工作来控制肌动蛋白动力学,并有助于解释细胞中肌动蛋白网络如何快速解聚。
更新日期:2019-11-22
中文翻译:

环化酶相关蛋白和 Cofilin 之间的协同作用可将肌动蛋白丝解聚加速两个数量级。
细胞肌动蛋白网络可以在几秒钟内快速分解和重塑,但体外肌动蛋白丝在几分钟内缓慢解聚。迄今为止,使肌动蛋白如此快速解聚的细胞机制仍不清楚。使用微流体辅助 TIRF,我们发现环化酶相关蛋白 (CAP) 和 Cofilin 协同作用,以比自发解聚快 330 倍的速度持续解聚肌动蛋白丝尖端。单分子成像进一步揭示,六聚体 CAP 分子与 Cofilin 修饰的细丝的尖端每次相互作用几秒钟,每次结合事件去除大约 100 个肌动蛋白亚基。这些发现建立了一个范例,其中丝末端结合蛋白和侧结合蛋白协同工作来控制肌动蛋白动力学,并有助于解释细胞中肌动蛋白网络如何快速解聚。































 京公网安备 11010802027423号
京公网安备 11010802027423号