当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Competition between Dissociation Pathway and Energy Transfer Pathway: Unimolecular Dissociation of a Benzene-Hexafluorobenzene Complex in Nitrogen Bath.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2019-12-06 , DOI: 10.1021/acs.jpca.9b07258 Sk Samir Ahamed 1 , Himashree Mahanta 1 , Amit K Paul 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2019-12-06 , DOI: 10.1021/acs.jpca.9b07258 Sk Samir Ahamed 1 , Himashree Mahanta 1 , Amit K Paul 1
Affiliation
|
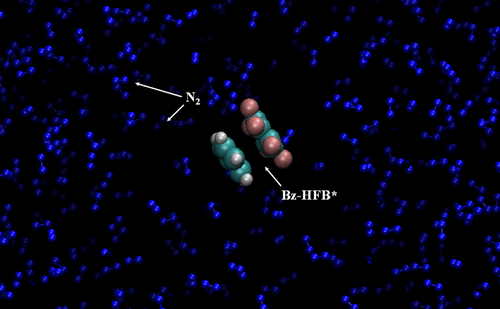
The unimolecular dissociation of a benzene-hexafluorobenzene complex at 1000, 1500, and 2000 K is studied inside a bath of 1000 N2 molecules kept at 300 K using chemical dynamics simulation. Three bath densities of 20, 324, and 750 kg/m3 are considered. The dissociation dynamics of the complex at a 20 kg/m3 bath density is found to be similar to that in the gas phase, whereas the dynamics is drastically different at higher bath densities. The microcanonical/canonical dissociation rate constants for the three bath densities are calculated and fitted to the Arrhenius equation. The activation energies are found to be similar to the gas-phase one. However, the pre-exponential factor is lower and decreases with the increase in bath density. The vibrational degree of freedom of the complex more effectively participates in the collisional energy transfer to the N2 bath, whereas the translational and rotational degrees of freedom of N2 receive the transferred energy. The energy transfer efficiency increases with the increase in bath density. The time scale of the energy transfer pathway is more than that of the dissociation pathway, and negligible direct dissociation of the complex is observed from the simulation at the highest bath density.
中文翻译:

解离途径与能量转移途径之间的竞争:氮气浴中苯-六氟苯配合物的单分子解离。
苯-六氟苯配合物在1000、1500和2000 K下的单分子解离作用是通过化学动力学模拟在1000 N2分子浴中保持300 K的条件下进行的。考虑了20、324和750 kg / m3的三种浴池密度。发现在20 kg / m3的熔池密度下,络合物的解离动力学与气相类似,而在较高的熔池密度下,动力学则完全不同。计算三种浴密度的微规范/规范解离速率常数,并将其拟合到Arrhenius方程。发现活化能类似于气相的活化能。但是,预指数因子较低,并且随着浴密度的增加而减小。配合物的振动自由度更有效地参与了向N2浴的碰撞能量转移,而N2的平移和旋转自由度则接收转移的能量。能量传递效率随着浴密度的增加而增加。能量转移途径的时间尺度大于解离途径的时间尺度,并且在最高浴密度下从模拟观察到复合物的可忽略的直接解离。
更新日期:2019-12-07
中文翻译:

解离途径与能量转移途径之间的竞争:氮气浴中苯-六氟苯配合物的单分子解离。
苯-六氟苯配合物在1000、1500和2000 K下的单分子解离作用是通过化学动力学模拟在1000 N2分子浴中保持300 K的条件下进行的。考虑了20、324和750 kg / m3的三种浴池密度。发现在20 kg / m3的熔池密度下,络合物的解离动力学与气相类似,而在较高的熔池密度下,动力学则完全不同。计算三种浴密度的微规范/规范解离速率常数,并将其拟合到Arrhenius方程。发现活化能类似于气相的活化能。但是,预指数因子较低,并且随着浴密度的增加而减小。配合物的振动自由度更有效地参与了向N2浴的碰撞能量转移,而N2的平移和旋转自由度则接收转移的能量。能量传递效率随着浴密度的增加而增加。能量转移途径的时间尺度大于解离途径的时间尺度,并且在最高浴密度下从模拟观察到复合物的可忽略的直接解离。































 京公网安备 11010802027423号
京公网安备 11010802027423号