当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Heterostructured intermetallic CuSn catalysts: high performance towards the electrochemical reduction of CO2 to formate†
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2019-11-22 , DOI: 10.1039/c9ta11140a
Jigang Wang 1, 2, 3, 4, 5 , Jiasui Zou 1, 2, 3, 4, 5 , Xiao Hu 1, 2, 3, 4, 5 , Shunlian Ning 1, 2, 3, 4, 5 , Xiujun Wang 3, 6, 7, 8 , Xiongwu Kang 1, 2, 3, 4, 5 , Shaowei Chen 9, 10, 11, 12
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2019-11-22 , DOI: 10.1039/c9ta11140a
Jigang Wang 1, 2, 3, 4, 5 , Jiasui Zou 1, 2, 3, 4, 5 , Xiao Hu 1, 2, 3, 4, 5 , Shunlian Ning 1, 2, 3, 4, 5 , Xiujun Wang 3, 6, 7, 8 , Xiongwu Kang 1, 2, 3, 4, 5 , Shaowei Chen 9, 10, 11, 12
Affiliation
|
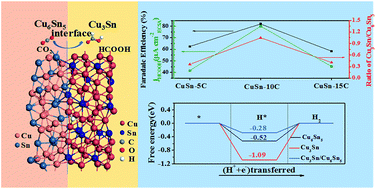
Electroreduction of carbon dioxide (CO2RR) into fuels and chemicals is an appealing approach to tackle CO2 emission challenges. To this end, it is critical to develop highly efficient and selective electrocatalysts for the CO2RR. Herein, we report a simple strategy for the preparation of heterostructured intermetallic CuSn electrocatalysts (Cu3Sn/Cu6Sn5) supported on porous copper foam through an electrodeposition–calcination process. The obtained CuSn intermetallic catalysts demonstrate a faradaic efficiency of 82% and a current density of 18.9 mA cm−2 at −1.0 V vs. the reversible hydrogen electrode for formate production in 0.1 M NaHCO3 electrolyte for as long as 42 h. By using a gas diffusion electrode and 1 M KOH electrolyte, the current density of this catalyst for formic acid production can reach values as high as 148 mA cm−2. Density functional theory calculations show that the moderate Gibbs free energy of hydrogen adsorption on the heterostructured Cu3Sn/Cu6Sn5 catalysts not only suppresses hydrogen evolution, but also favors the production of formic acid. This study demonstrates a straightforward approach to the preparation of high-performance electrocatalysts towards the selective electroreduction of CO2 to formate.
中文翻译:

异质结构金属间化合物CuSn催化剂:高性能,可将CO 2电化学还原成甲酸酯†
将二氧化碳(CO 2 RR)电还原为燃料和化学品是解决CO 2排放挑战的一种有吸引力的方法。为此,开发用于CO 2 RR的高效且选择性的电催化剂至关重要。本文中,我们报告了一种通过电沉积-煅烧工艺制备负载在多孔泡沫铜上的异质结构金属间CuSn电催化剂(Cu 3 Sn / Cu 6 Sn 5)的简单策略。将所得的CuSn金属间催化剂表明82%的法拉第效率和18.9毫安cm 2的电流密度-2在的-1.0V与在0.1 M NaHCO 3电解液中甲酸生成的可逆氢电极长达42 h。通过使用气体扩散电极和1M KOH电解质,该用于甲酸生产的催化剂的电流密度可以达到高达148mA cm -2的值。密度泛函理论计算表明,异结构Cu 3 Sn / Cu 6 Sn 5催化剂上适度的氢吸附吉布斯自由能不仅抑制氢的释放,而且有利于甲酸的产生。这项研究证明了一种直接制备高性能电催化剂的方法,可以选择性地将CO 2电还原为甲酸。
更新日期:2019-12-11
中文翻译:

异质结构金属间化合物CuSn催化剂:高性能,可将CO 2电化学还原成甲酸酯†
将二氧化碳(CO 2 RR)电还原为燃料和化学品是解决CO 2排放挑战的一种有吸引力的方法。为此,开发用于CO 2 RR的高效且选择性的电催化剂至关重要。本文中,我们报告了一种通过电沉积-煅烧工艺制备负载在多孔泡沫铜上的异质结构金属间CuSn电催化剂(Cu 3 Sn / Cu 6 Sn 5)的简单策略。将所得的CuSn金属间催化剂表明82%的法拉第效率和18.9毫安cm 2的电流密度-2在的-1.0V与在0.1 M NaHCO 3电解液中甲酸生成的可逆氢电极长达42 h。通过使用气体扩散电极和1M KOH电解质,该用于甲酸生产的催化剂的电流密度可以达到高达148mA cm -2的值。密度泛函理论计算表明,异结构Cu 3 Sn / Cu 6 Sn 5催化剂上适度的氢吸附吉布斯自由能不仅抑制氢的释放,而且有利于甲酸的产生。这项研究证明了一种直接制备高性能电催化剂的方法,可以选择性地将CO 2电还原为甲酸。

































 京公网安备 11010802027423号
京公网安备 11010802027423号