Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interaction of Phosphate with Lithium Cobalt Oxide Nanoparticles: A Combined Spectroscopic and Calorimetric Study.
Langmuir ( IF 3.7 ) Pub Date : 2019-12-05 , DOI: 10.1021/acs.langmuir.9b02708 Elizabeth D Laudadio 1 , Poorandokht Ilani-Kashkouli , Curtis M Green 1 , Nadine J Kabengi , Robert J Hamers 1
Langmuir ( IF 3.7 ) Pub Date : 2019-12-05 , DOI: 10.1021/acs.langmuir.9b02708 Elizabeth D Laudadio 1 , Poorandokht Ilani-Kashkouli , Curtis M Green 1 , Nadine J Kabengi , Robert J Hamers 1
Affiliation
|
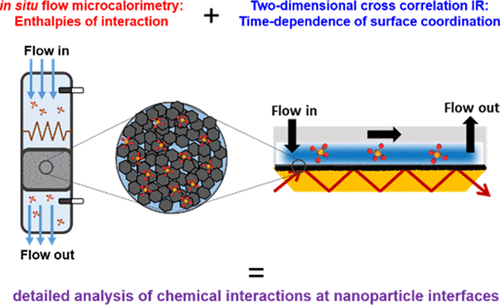
Adsorption of small ions such as phosphates to the surfaces of metal oxides can significantly alter the behavior of these materials, especially when present in the nanoscale form. Lithium cobalt oxide is a good model system for understanding small-molecule interactions with emerging nanomaterials because of its widespread use in lithium ion batteries and its known activity as a water oxidation catalyst. Here, we present a thermodynamic analysis of phosphate adsorption to LiCoO2 and corroborate the results with additional in situ techniques, including zeta potential measurements and attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy, at pH values relevant to potential environmental release scenarios. Flow microcalorimetry measurements of phosphate interaction with LiCoO2 at pH 7.4 show that there are two distinct exothermic processes taking place. Time-sequence in situ ATR-FTIR with two-dimensional correlation analysis reveals the spectroscopic signatures of these processes. We interpret the data as an interaction of phosphate with LiCoO2 that occurs through the release of two water molecules and is therefore, best described as a condensation process rather than a simple adsorption, consistent with prior studies, demonstrating that phosphate interaction with LiCoO2 is highly irreversible. Additional measurements for over longer times of 5 months show that phosphate adsorption terminates with one surface layer and that continued transformation over longer periods of time arises from H+/Li+ exchange and slow transformation to a cobalt hydroxide, with phosphate adsorbed to the surface only. To the best of our knowledge, this is the first time that flow microcalorimetry and two-dimensional correlation spectroscopy have been applied in tandem to clarify the specific chemical reactions that occur at the interface of solids and adsorbates.
中文翻译:

磷酸盐与钴酸锂纳米粒子的相互作用:光谱和量热研究的结合。
小离子(例如磷酸根)吸附到金属氧化物的表面会显着改变这些材料的行为,尤其是当以纳米级形式存在时。钴酸锂是一种很好的模型系统,可用于理解与新兴纳米材料的小分子相互作用,这是因为其在锂离子电池中的广泛使用以及其作为水氧化催化剂的已知活性。在这里,我们介绍了磷酸盐吸附到LiCoO2上的热力学分析,并通过其他原位技术(包括zeta电位测量和衰减的全反射傅里叶变换红外(ATR-FTIR)光谱)在与潜在环境释放场景相关的pH值下证实了结果。 。流动微法测量pH值为7时磷酸盐与LiCoO2的相互作用。图4表明发生了两种不同的放热过程。具有二维相关性分析的时间顺序原位ATR-FTIR揭示了这些过程的光谱特征。我们将数据解释为磷酸盐与LiCoO2的相互作用是通过释放两个水分子而发生的,因此,最好将其描述为缩合过程,而不是简单的吸附,这与先前的研究一致,表明磷酸盐与LiCoO2的相互作用是高度不可逆的。 。在5个月的较长时间内进行的其他测量表明,磷酸盐吸附终止于一个表面层,并且在较长时间段内的连续转化是由于H + / Li +交换和缓慢转化为氢氧化钴而产生的,而磷酸盐仅吸附在表面上。据我们所知,
更新日期:2019-12-06
中文翻译:

磷酸盐与钴酸锂纳米粒子的相互作用:光谱和量热研究的结合。
小离子(例如磷酸根)吸附到金属氧化物的表面会显着改变这些材料的行为,尤其是当以纳米级形式存在时。钴酸锂是一种很好的模型系统,可用于理解与新兴纳米材料的小分子相互作用,这是因为其在锂离子电池中的广泛使用以及其作为水氧化催化剂的已知活性。在这里,我们介绍了磷酸盐吸附到LiCoO2上的热力学分析,并通过其他原位技术(包括zeta电位测量和衰减的全反射傅里叶变换红外(ATR-FTIR)光谱)在与潜在环境释放场景相关的pH值下证实了结果。 。流动微法测量pH值为7时磷酸盐与LiCoO2的相互作用。图4表明发生了两种不同的放热过程。具有二维相关性分析的时间顺序原位ATR-FTIR揭示了这些过程的光谱特征。我们将数据解释为磷酸盐与LiCoO2的相互作用是通过释放两个水分子而发生的,因此,最好将其描述为缩合过程,而不是简单的吸附,这与先前的研究一致,表明磷酸盐与LiCoO2的相互作用是高度不可逆的。 。在5个月的较长时间内进行的其他测量表明,磷酸盐吸附终止于一个表面层,并且在较长时间段内的连续转化是由于H + / Li +交换和缓慢转化为氢氧化钴而产生的,而磷酸盐仅吸附在表面上。据我们所知,













































 京公网安备 11010802027423号
京公网安备 11010802027423号