Water Research ( IF 11.4 ) Pub Date : 2018-03-12 , DOI: 10.1016/j.watres.2018.03.030 Jianbing Wang , Dan Zhi , Hao Zhou , Xuwen He , Dayi Zhang
|
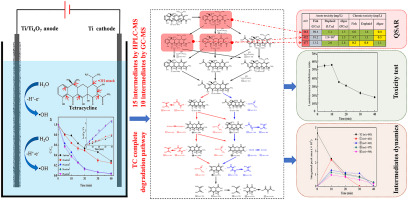
Tetracycline (TC) is one of the most widely used antibiotics with significant impacts on human health and thus it needs appropriate approaches for its removal. In the present study, we evaluated the performance and complete pathway of the TC electrochemical oxidation on a Ti/Ti4O7 anode prepared by plasma spraying. Morphological data and composition analysis indicated a compact coating layer on the anode, which had the characteristic peaks of Ti4O7 as active constituent. The TC electrochemical oxidation on the Ti/Ti4O7 anode followed a pseudo-first-order kinetics, and the TC removal efficiency reached 95.8% in 40 min. The influential factors on TC decay kinetics included current density, anode-cathode distance and initial TC concentration. This anode also had high durability and the TC removal efficiency was maintained over 95% after five times reuse. For the first time, we unraveled the complete pathway of the TC electrochemical oxidation using high-performance liquid chromatograph (HPLC) and gas chromatograph (GC) coupled with mass spectrometer (MS). ·OH radicals produced from electrochemical oxidation attack the double bond, phenolic group and amine group of TC, forming a primary intermediate (m/z = 461), secondary intermediates (m/z = 432, 477 and 509) and tertiary intermediates (m/z = 480, 448 and 525). The latter were further oxidized to the key downstream intermediate (m/z = 496), followed by further downstream intermediates (m/z = 451, 412, 396, 367, 351, 298 and 253) and eventually short-chain carboxylic acids. We also evaluated the toxicity change during the electrochemical oxidation process with bioluminescent bacteria. The bioluminescence inhibition ratio peaked at 10 min (55.41%), likely owing to the high toxicity of intermediates with m/z = 461, 432 and 477 as obtained from quantitative structure activity relationship (QSAR) analysis. The bioluminescence inhibition rate eventually decreased to 16.78% in 40 min due to further transformation of TC and intermediates. By comprehensively analyzing the influential factors and complete degradation pathway of TC electrochemical oxidation on the Ti/Ti4O7 anode, our research provides deeper insights into the risk assessment of intermediates and their toxicity, assigning new perspectives for practical electrochemical oxidation to effectively eliminate the amount and toxicity of TC and other antibiotics in wastewater.
中文翻译:

在Ti / Ti 4 O 7阳极上电化学氧化过程中评估四环素降解途径和中间毒性
四环素(TC)是使用最广泛的抗生素之一,对人体健康有重大影响,因此需要适当的方法将其清除。在本研究中,我们评估了通过等离子喷涂制备的Ti / Ti 4 O 7阳极上TC电化学氧化的性能和完整途径。形态数据和组成分析表明阳极上有致密的涂层,该涂层具有特征峰Ti 4 O 7作为活性成分。Ti / Ti 4 O 7上的TC电化学氧化阳极遵循拟一阶动力学,TC去除效率在40分钟内达到95.8%。影响TC衰减动力学的因素包括电流密度,阳极-阴极距离和初始TC浓度。该阳极还具有高耐久性,并且在重复使用五次后,TC去除效率保持在95%以上。我们首次使用高效液相色谱仪(HPLC)和气相色谱仪(GC)结合质谱仪(MS)揭示了TC电化学氧化的完整途径。·电化学氧化产生的OH自由基攻击TC的双键,酚基和胺基,形成一级中间体(m / z = 461),二级中间体(m / z = 432、477和509)和叔中间体(m / z = 480、448和525)。后者被进一步氧化成关键的下游中间体(m / z = 496),然后被进一步的下游中间体(m / z = 451、412、396、367、351、298和253)氧化,最终成为短链羧酸。我们还评估了生物发光细菌在电化学氧化过程中的毒性变化。生物发光抑制率在10分钟时达到峰值(55.41%),这可能是由于m / z中间体的高毒性 通过定量结构活度关系(QSAR)分析获得的461、432和477。由于TC和中间体的进一步转化,生物发光抑制率最终在40分钟内降低到16.78%。通过全面分析Ti / Ti 4 O 7阳极上TC电化学氧化的影响因素和完整的降解途径,我们的研究为中间体风险及其毒性的评估提供了更深刻的见解,为实际电化学氧化提供了新的见解,从而有效地消除了中间产物。废水中TC和其他抗生素的含量和毒性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号