当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
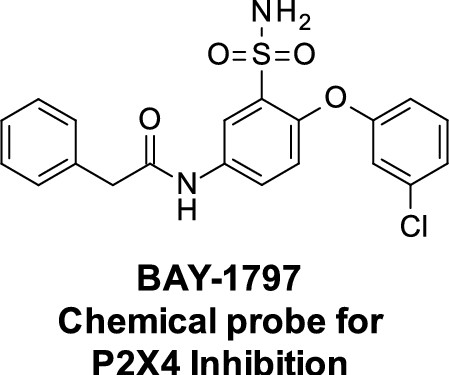
Discovery and Characterization of the Potent and Selective P2X4 Inhibitor N-[4-(3-Chlorophenoxy)-3-sulfamoylphenyl]-2-phenylacetamide (BAY-1797) and Structure-Guided Amelioration of Its CYP3A4 Induction Profile.
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2019-12-11 , DOI: 10.1021/acs.jmedchem.9b01304 Stefan Werner 1 , Stefanie Mesch 1 , Roman C Hillig 1 , Antonius Ter Laak 1 , Julie Klint 2 , Ioana Neagoe 2 , Alexis Laux-Biehlmann 1 , Henrik Dahllöf 1 , Nico Bräuer 1 , Vera Puetter 1 , Reinhard Nubbemeyer 1 , Simone Schulz 1 , Michaela Bairlein 1 , Thomas M Zollner 1 , Andreas Steinmeyer 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2019-12-11 , DOI: 10.1021/acs.jmedchem.9b01304 Stefan Werner 1 , Stefanie Mesch 1 , Roman C Hillig 1 , Antonius Ter Laak 1 , Julie Klint 2 , Ioana Neagoe 2 , Alexis Laux-Biehlmann 1 , Henrik Dahllöf 1 , Nico Bräuer 1 , Vera Puetter 1 , Reinhard Nubbemeyer 1 , Simone Schulz 1 , Michaela Bairlein 1 , Thomas M Zollner 1 , Andreas Steinmeyer 1
Affiliation
|
The P2X4 receptor is a ligand-gated ion channel that is expressed on a variety of cell types, especially those involved in inflammatory and immune processes. High-throughput screening led to a new class of P2X4 inhibitors with substantial CYP 3A4 induction in human hepatocytes. A structure-guided optimization with respect to decreased pregnane X receptor (PXR) binding was started. It was found that the introduction of larger and more polar substituents on the ether linker led to less PXR binding while maintaining the P2X4 inhibitory potency. This translated into significantly reduced CYP 3A4 induction for compounds 71 and 73. Unfortunately, the in vivo pharmacokinetic (PK) profiles of these compounds were insufficient for the desired profile in humans. However, BAY-1797 (10) was identified and characterized as a potent and selective P2X4 antagonist. This compound is suitable for in vivo studies in rodents, and the anti-inflammatory and anti-nociceptive effects of BAY-1797 were demonstrated in a mouse complete Freund's adjuvant (CFA) inflammatory pain model.
中文翻译:

有效和选择性P2X4抑制剂N- [4-(3-氯苯氧基)-3-氨磺酰基苯基] -2-苯基乙酰胺(BAY-1797)的发现和表征,以及其CYP3A4诱导曲线的结构指导改进。
P2X4受体是配体门控的离子通道,可在多种细胞类型中表达,尤其是与炎症和免疫过程有关的细胞。高通量筛选导致一类新的P2X4抑制剂在人肝细胞中具有实质性的CYP 3A4诱导作用。开始针对减少的孕烷X受体(PXR)结合进行结构指导的优化。发现在醚接头上引入更大和更多极性的取代基导致更少的PXR结合,同时保持P2X4抑制能力。这转化为对化合物71和73的CYP 3A4诱导作用明显降低。不幸的是,这些化合物的体内药代动力学(PK)谱不足以达到人体所需的谱。然而,BAY-1797(10)被鉴定为有效的选择性P2X4拮抗剂。该化合物适合在啮齿动物中进行体内研究,并且在小鼠完全弗氏佐剂(CFA)炎性疼痛模型中证明了BAY-1797的抗炎和抗伤害感受作用。
更新日期:2019-12-13
中文翻译:

有效和选择性P2X4抑制剂N- [4-(3-氯苯氧基)-3-氨磺酰基苯基] -2-苯基乙酰胺(BAY-1797)的发现和表征,以及其CYP3A4诱导曲线的结构指导改进。
P2X4受体是配体门控的离子通道,可在多种细胞类型中表达,尤其是与炎症和免疫过程有关的细胞。高通量筛选导致一类新的P2X4抑制剂在人肝细胞中具有实质性的CYP 3A4诱导作用。开始针对减少的孕烷X受体(PXR)结合进行结构指导的优化。发现在醚接头上引入更大和更多极性的取代基导致更少的PXR结合,同时保持P2X4抑制能力。这转化为对化合物71和73的CYP 3A4诱导作用明显降低。不幸的是,这些化合物的体内药代动力学(PK)谱不足以达到人体所需的谱。然而,BAY-1797(10)被鉴定为有效的选择性P2X4拮抗剂。该化合物适合在啮齿动物中进行体内研究,并且在小鼠完全弗氏佐剂(CFA)炎性疼痛模型中证明了BAY-1797的抗炎和抗伤害感受作用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号