当前位置:
X-MOL 学术
›
Arab. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quartzite an efficient adsorbent for the removal of anionic and cationic dyes from aqueous solutions
Arabian Journal of Chemistry ( IF 5.3 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.arabjc.2019.11.001 Shah Hussain , Noor ul Amin , Shahid Ali Khan
Arabian Journal of Chemistry ( IF 5.3 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.arabjc.2019.11.001 Shah Hussain , Noor ul Amin , Shahid Ali Khan
|
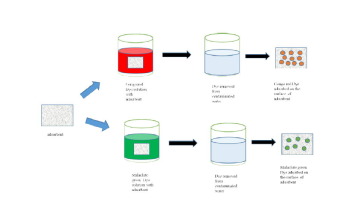
Abstract Quartzite obtained from local source was investigated for the removal of anionic dye congo red (CR) and cationic dye malachite green (MG) as an adsorbent from aqueous solution in batch experiment. The adsorption process was studied as a function of dye concentration, contact time, pH and temperature. Adsorption process was described well by Langmuir and Freundlich isotherms. The adsorption capacity remained 666.7 mg/g for CR dye and 348.125 mg/g for MG dye. Data was analyzed thermodynamically, ΔH0 and ΔG0 values proved that adsorption of CR and MG is an endothermic and spontaneous process. Adsorption data fitted best in the pseudo-first order kinetic model. The adsorption data proved that quartzite exhibits the best adsorption capacity and can be utilized for the removal of anionic and cationic dyes.
中文翻译:

石英岩是一种有效的吸附剂,用于从水溶液中去除阴离子和阳离子染料
摘要 在批量实验中,研究了从当地获得的石英岩从水溶液中去除作为吸附剂的阴离子染料刚果红(CR)和阳离子染料孔雀石绿(MG)。吸附过程被研究为染料浓度、接触时间、pH 值和温度的函数。Langmuir 和 Freundlich 等温线很好地描述了吸附过程。CR 染料的吸附容量保持为 666.7 mg/g,MG 染料的吸附容量保持为 348.125 mg/g。对数据进行热力学分析,ΔH0和ΔG0值证明CR和MG的吸附是吸热和自发过程。吸附数据最适合伪一级动力学模型。吸附数据证明石英岩表现出最好的吸附能力,可用于去除阴离子和阳离子染料。
更新日期:2020-03-01
中文翻译:

石英岩是一种有效的吸附剂,用于从水溶液中去除阴离子和阳离子染料
摘要 在批量实验中,研究了从当地获得的石英岩从水溶液中去除作为吸附剂的阴离子染料刚果红(CR)和阳离子染料孔雀石绿(MG)。吸附过程被研究为染料浓度、接触时间、pH 值和温度的函数。Langmuir 和 Freundlich 等温线很好地描述了吸附过程。CR 染料的吸附容量保持为 666.7 mg/g,MG 染料的吸附容量保持为 348.125 mg/g。对数据进行热力学分析,ΔH0和ΔG0值证明CR和MG的吸附是吸热和自发过程。吸附数据最适合伪一级动力学模型。吸附数据证明石英岩表现出最好的吸附能力,可用于去除阴离子和阳离子染料。





























 京公网安备 11010802027423号
京公网安备 11010802027423号