Journal of CO2 Utilization ( IF 7.2 ) Pub Date : 2019-11-19 , DOI: 10.1016/j.jcou.2019.11.005 Yongseok Kim , Seungdon Kwon , Yohan Song , Kyungsu Na
|
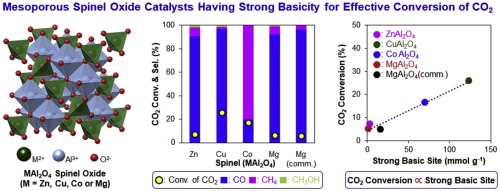
CO2 hydrogenation using H2 was investigated using mesoporous bimetallic aluminum spinel oxides (MAl2O4, where M was Mg, Co, Cu, or Zn) as heterogeneous base catalysts. They could catalyze CO2 hydrogenation without additional catalytic metal components. Most catalysts produced CO as the major product (>80%) and relatively small amounts of CH4 and CH3OH. However, when CoAl2O4 was used as catalyst, CH4 was the major product (>80%). A systematic relationship was discovered between the amount of strong basic sites and CO2 conversion. As the amount of strong basic sites increased, the CO2 conversion increased with a linear relationship over all the tested reaction temperatures (300–400 °C). However, no systematic relationship was observed between the CO2 conversion and total amount of basic sites, which suggested that only the sufficiently strong basic sites could activate CO2 efficiently. In addition, owing to the high surface area of the mesopores, the catalytic activity of the spinel oxides increased because of the facile diffusion of molecules through their mesoporous channels. Assuming that the strong basic sites were the actual catalytic sites, the apparent activation energies of all catalysts were derived. The results indicated that CuAl2O4 having the highest amount of strong basic sites presented the lowest activation energy of 13.0 kJ mol−1. This reemphasized the idea that the strong basic sites were useful for the effective activation of CO2 with small energy input. Accordingly, these spinel oxides could be further used for designing multifunctional catalysts supporting catalytic metal nanoparticles on their mesopore walls, which is under investigation.
中文翻译:

使用介孔双金属尖晶石氧化物作为活性多相碱催化剂,具有长寿命的催化CO 2加氢
使用介孔双金属铝尖晶石氧化物(MAl 2 O 4,其中M为Mg,Co,Cu或Zn)作为多相碱催化剂,研究了使用H 2进行CO 2加氢。它们可以催化CO 2加氢而无需其他催化金属组分。大多数催化剂产生的主要产物为CO(> 80%)和相对少量的CH 4和CH 3 OH。然而,当使用CoAl 2 O 4作为催化剂时,CH 4是主要产物(> 80%)。发现强碱位的量与CO 2之间存在系统关系转换。随着强碱性位点数量的增加,在所有测试的反应温度(300–400°C)下,CO 2转化率呈线性关系增加。然而,在CO 2转化率与碱性位点总量之间没有观察到系统的关系,这表明只有足够强的碱性位点才能有效地激活CO 2。另外,由于介孔的表面积大,由于分子易于通过其介孔通道扩散,尖晶石氧化物的催化活性增加。假定强碱性位点是实际的催化位点,则得出所有催化剂的表观活化能。结果表明,CuAl 2 O4具有强碱性位的最高量呈现13.0千焦摩尔的最低活化能-1。这再次强调了这样的想法,即强碱性位点对于以小能量输入有效激活CO 2是有用的。因此,这些尖晶石氧化物可进一步用于设计在其中孔壁上担载催化金属纳米颗粒的多功能催化剂,这正在研究中。






























 京公网安备 11010802027423号
京公网安备 11010802027423号