当前位置:
X-MOL 学术
›
Int. J. Quantum Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self‐catalyzed keto‐enol tautomerization of malonic acid
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2019-11-20 , DOI: 10.1002/qua.26114 Catherine C. R. Sutton 1 , Chia‐Yang Lim 1 , Gabriel Silva 1
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2019-11-20 , DOI: 10.1002/qua.26114 Catherine C. R. Sutton 1 , Chia‐Yang Lim 1 , Gabriel Silva 1
Affiliation
|
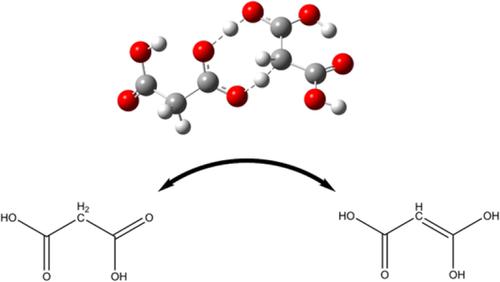
We demonstrate through quantum chemical calculations that the keto-enol tautomerization of malonic acid can be catalyzed by the two tautomers of malonic acid itself. This self-catalyzed process proceeds with a relatively low barrier (Gibbs energy ca. 13 kcal/mol in gas phase, 20 kcal/mol in aqueous phase), and involves the concerted transfer of two protons between the substrate and the carboxylic acid functionality of the malonic acid catalyst. This mechanism is expected to compete with the proton relay mechanism currently favored to explain the tautomerization of malonic acid in aqueous media. Malonic acid is an important constituent of secondary organic aerosol where the present chemistry may play a role in determining chemical composition.
中文翻译:

丙二酸的自催化酮-烯醇互变异构化
我们通过量子化学计算证明丙二酸的酮-烯醇互变异构可以由丙二酸本身的两个互变异构体催化。这种自催化过程以相对较低的势垒进行(气相中吉布斯能量约为 13 kcal/mol,水相中约为 20 kcal/mol),并且涉及两个质子在底物和羧酸官能团之间的协同转移。丙二酸催化剂。预计这种机制将与目前用于解释丙二酸在水介质中互变异构的质子中继机制竞争。丙二酸是二次有机气溶胶的重要成分,其中本化学可能在确定化学成分方面发挥作用。
更新日期:2019-11-20
中文翻译:

丙二酸的自催化酮-烯醇互变异构化
我们通过量子化学计算证明丙二酸的酮-烯醇互变异构可以由丙二酸本身的两个互变异构体催化。这种自催化过程以相对较低的势垒进行(气相中吉布斯能量约为 13 kcal/mol,水相中约为 20 kcal/mol),并且涉及两个质子在底物和羧酸官能团之间的协同转移。丙二酸催化剂。预计这种机制将与目前用于解释丙二酸在水介质中互变异构的质子中继机制竞争。丙二酸是二次有机气溶胶的重要成分,其中本化学可能在确定化学成分方面发挥作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号