当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Collision Induced Unfolding Classifies Ligands Bound to the Integral Membrane Translocator Protein.
Analytical Chemistry ( IF 6.7 ) Pub Date : 2019-12-05 , DOI: 10.1021/acs.analchem.9b03208 Sarah M Fantin 1 , Kristine F Parson 1 , Shuai Niu 1 , Jian Liu 2 , Daniel A Polasky 1 , Sugyan M Dixit 1 , Shelagh M Ferguson-Miller 2 , Brandon T Ruotolo 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2019-12-05 , DOI: 10.1021/acs.analchem.9b03208 Sarah M Fantin 1 , Kristine F Parson 1 , Shuai Niu 1 , Jian Liu 2 , Daniel A Polasky 1 , Sugyan M Dixit 1 , Shelagh M Ferguson-Miller 2 , Brandon T Ruotolo 1
Affiliation
|
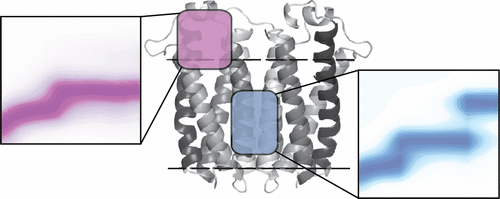
Membrane proteins represent most current therapeutic targets, yet remain understudied due to their insolubility in aqueous solvents and generally low yields during purification and expression. Ion mobility-mass spectrometry and collision induced unfolding experiments have recently garnered attention as methods capable of directly detecting and quantifying ligand binding within a wide range of membrane protein systems. Despite prior success, ionized surfactant often creates chemical noise patterns resulting in significant challenges surrounding the study of small membrane protein-ligand complexes. Here, we present a new data analysis workflow that overcomes such chemical noise and then utilize this approach to quantify and classify ligand binding associated with the 36 kDa dimer of translocator protein (TSPO). Following our denoising protocol, we detect separate gas-phase unfolding signatures for lipid and protoporphyrin TSPO binders, molecular classes that likely interact with separate regions of the protein surface. Further, a detailed classification analysis reveals that lipid alkyl chain saturation levels can be detected within our gas-phase protein unfolding data. We combine these data and classification schemes with mass spectra acquired directly from liquid-liquid extracts to propose an identity for a previously unknown endogenous TSPO ligand.
中文翻译:

碰撞诱导的展开将结合的配体分类为完整的膜转运蛋白。
膜蛋白代表了目前的大多数治疗靶标,但由于它们在水性溶剂中的不溶性以及纯化和表达过程中的低产率而仍未被充分研究。离子淌度质谱和碰撞诱导的展开实验最近作为一种能够直接检测和定量各种膜蛋白系统中配体结合的方法而受到关注。尽管取得了先前的成功,但离子化表面活性剂通常会产生化学噪声模式,从而导致围绕小膜蛋白-配体复合物研究的重大挑战。在这里,我们提出了一种新的数据分析工作流程,该工作流程克服了这种化学噪音,然后利用这种方法来量化和分类与转运蛋白(TSPO)的36 kDa二聚体相关的配体结合。按照我们的降噪协议,我们检测到脂类和原卟啉TSPO粘合剂的独立气相展开特征,这些分子类别可能与蛋白质表面的不同区域相互作用。此外,详细的分类分析表明,在我们的气相蛋白质展开数据中可以检测到脂质烷基链饱和度水平。我们将这些数据和分类方案与直接从液-液提取物中获得的质谱相结合,以提出先前未知的内源TSPO配体的身份。
更新日期:2019-12-05
中文翻译:

碰撞诱导的展开将结合的配体分类为完整的膜转运蛋白。
膜蛋白代表了目前的大多数治疗靶标,但由于它们在水性溶剂中的不溶性以及纯化和表达过程中的低产率而仍未被充分研究。离子淌度质谱和碰撞诱导的展开实验最近作为一种能够直接检测和定量各种膜蛋白系统中配体结合的方法而受到关注。尽管取得了先前的成功,但离子化表面活性剂通常会产生化学噪声模式,从而导致围绕小膜蛋白-配体复合物研究的重大挑战。在这里,我们提出了一种新的数据分析工作流程,该工作流程克服了这种化学噪音,然后利用这种方法来量化和分类与转运蛋白(TSPO)的36 kDa二聚体相关的配体结合。按照我们的降噪协议,我们检测到脂类和原卟啉TSPO粘合剂的独立气相展开特征,这些分子类别可能与蛋白质表面的不同区域相互作用。此外,详细的分类分析表明,在我们的气相蛋白质展开数据中可以检测到脂质烷基链饱和度水平。我们将这些数据和分类方案与直接从液-液提取物中获得的质谱相结合,以提出先前未知的内源TSPO配体的身份。






























 京公网安备 11010802027423号
京公网安备 11010802027423号