当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of Chiral Vicinal Amino Alcohols Using Amine Dehydrogenases
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-11-25 , DOI: 10.1021/acscatal.9b03889 Fei-Fei Chen 1, 2 , Sebastian C. Cosgrove 2 , William R. Birmingham 2 , Juan Mangas-Sanchez 2 , Joan Citoler 2 , Matthew P. Thompson 2 , Gao-Wei Zheng 1 , Jian-He Xu 1 , Nicholas J. Turner 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-11-25 , DOI: 10.1021/acscatal.9b03889 Fei-Fei Chen 1, 2 , Sebastian C. Cosgrove 2 , William R. Birmingham 2 , Juan Mangas-Sanchez 2 , Joan Citoler 2 , Matthew P. Thompson 2 , Gao-Wei Zheng 1 , Jian-He Xu 1 , Nicholas J. Turner 2
Affiliation
|
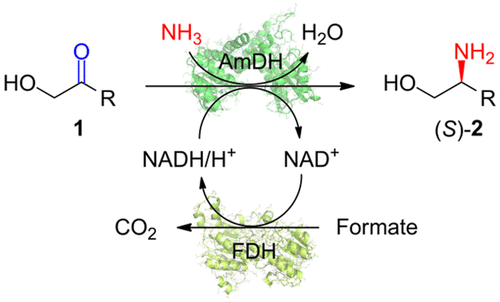
Chiral vicinal amino alcohols are an important motif found in many biologically active molecules. In this study, biocatalytic reductive amination of α-hydroxy ketones with ammonia was investigated using engineered amine dehydrogenases (AmDHs) derived from the leucine amino acid dehydrogenase (AADH) from Lysinibacillus fusiformis. The AmDHs thus identified enabled the synthesis of (S)-configured vicinal amino alcohols from the corresponding α-hydroxy ketones in up to 99% conversions and >99% ee. One of the AmDH variants was used to prepare a key intermediate for the antituberculosis pharmaceutical ethambutol.
中文翻译:

使用胺脱氢酶的手性邻氨基醇的对映选择性合成
手性邻氨基氨基醇是在许多生物活性分子中发现的重要基序。在这项研究中,使用衍生自梭状芽孢杆菌的亮氨酸氨基酸脱氢酶(AADH)的工程胺脱氢酶(AmDHs),研究了α-羟基酮与氨的生物催化还原胺化反应。因此鉴定出的AmDHs能够以高达99%的转化率和> 99%的ee从相应的α-羟基酮合成(S)-构型的邻氨基氨基醇。AmDH变体之一用于制备抗结核药物乙胺丁醇的关键中间体。
更新日期:2019-11-28
中文翻译:

使用胺脱氢酶的手性邻氨基醇的对映选择性合成
手性邻氨基氨基醇是在许多生物活性分子中发现的重要基序。在这项研究中,使用衍生自梭状芽孢杆菌的亮氨酸氨基酸脱氢酶(AADH)的工程胺脱氢酶(AmDHs),研究了α-羟基酮与氨的生物催化还原胺化反应。因此鉴定出的AmDHs能够以高达99%的转化率和> 99%的ee从相应的α-羟基酮合成(S)-构型的邻氨基氨基醇。AmDH变体之一用于制备抗结核药物乙胺丁醇的关键中间体。





























 京公网安备 11010802027423号
京公网安备 11010802027423号