当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantiodivergence by minimal modification of an acyclic chiral secondary aminocatalyst.
Nature Communications ( IF 14.7 ) Pub Date : 2019-11-15 , DOI: 10.1038/s41467-019-13183-5
Jun Dai 1 , Zhuang Wang 1 , Yuhua Deng 1 , Lei Zhu 2 , Fangzhi Peng 1 , Yu Lan 2 , Zhihui Shao 1
Nature Communications ( IF 14.7 ) Pub Date : 2019-11-15 , DOI: 10.1038/s41467-019-13183-5
Jun Dai 1 , Zhuang Wang 1 , Yuhua Deng 1 , Lei Zhu 2 , Fangzhi Peng 1 , Yu Lan 2 , Zhihui Shao 1
Affiliation
|
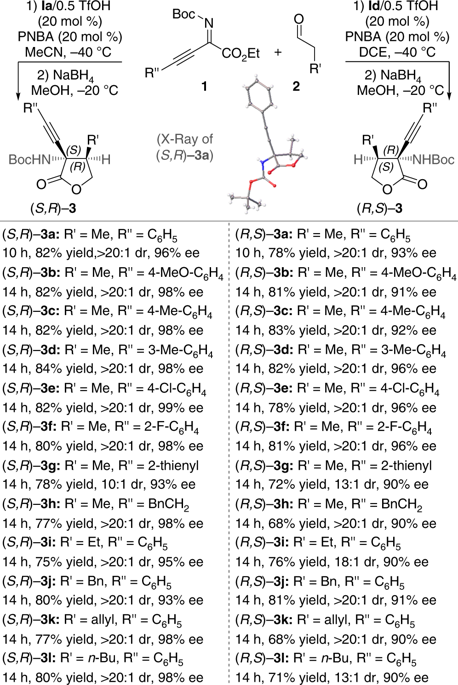
The development of enantiodivergent catalysis for the preparation of both enantiomers of a chiral compound is of importance in pharmaceutical and bioorganic chemistry. With the design of a class of reactive and stereoselective organocatalysts, acyclic chiral secondary amines, a method for achieving the enantiodivergence is developed simply by changing the secondary N-i-Bu- to N-Me-group within the catalyst architecture while maintaining the same absolute configuration of the catalysts, which modulates the catalyst conformation. This catalyst-controlled enantiodivergent method not only enables challenging asymmetric transformations to occur in an enantiodivergent manner but also features a high level of stereocontrol and broad scope that is demonstrated in eight different reactions (90 examples), all delivering both enantiomers of a range of structurally diverse products including hitherto less accessible, yet important, compounds in good yields with high stereoselectivities.
中文翻译:

通过最小程度地修饰无环手性仲氨基催化剂进行对映异构。
开发用于制备手性化合物的两种对映异构体的对映异构体催化在药物和生物有机化学中是重要的。通过设计一类反应性和立体选择性有机催化剂,即无环手性仲胺,只需在保持相同的绝对构型的同时将催化剂中的仲Ni-Bu-改为N-Me-即可简单地开发出一种用于实现对映异构的方法。调节催化剂的构象。这种催化剂控制的对映体发散方法不仅能够以对映体的方式进行具有挑战性的不对称转化,而且还具有高水平的立体控制和广泛的范围,这在八个不同的反应中得到了证明(90个实例),
更新日期:2019-11-15
中文翻译:

通过最小程度地修饰无环手性仲氨基催化剂进行对映异构。
开发用于制备手性化合物的两种对映异构体的对映异构体催化在药物和生物有机化学中是重要的。通过设计一类反应性和立体选择性有机催化剂,即无环手性仲胺,只需在保持相同的绝对构型的同时将催化剂中的仲Ni-Bu-改为N-Me-即可简单地开发出一种用于实现对映异构的方法。调节催化剂的构象。这种催化剂控制的对映体发散方法不仅能够以对映体的方式进行具有挑战性的不对称转化,而且还具有高水平的立体控制和广泛的范围,这在八个不同的反应中得到了证明(90个实例),

































 京公网安备 11010802027423号
京公网安备 11010802027423号