当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactivity and Mechanisms in Fluoride Shuttle Battery Reactions: Difference between Orthorhombic and Cubic BiF3 Single Microparticles
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2019-11-14 00:00:00 , DOI: 10.1021/acsaem.9b01803 Toshiro Yamanaka 1 , Ken-ichi Okazaki 1 , Zempachi Ogumi 1 , Takeshi Abe 2
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2019-11-14 00:00:00 , DOI: 10.1021/acsaem.9b01803 Toshiro Yamanaka 1 , Ken-ichi Okazaki 1 , Zempachi Ogumi 1 , Takeshi Abe 2
Affiliation
|
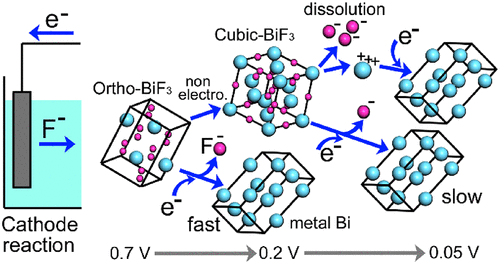
BiF3 is a strong candidate for a cathode material in fluoride shuttle batteries (FSBs), which utilize defluorination of metal fluorides and fluorination of metals. Much interest has been shown in FSBs as next-generation batteries with high energy densities. Elucidation and control of reactivity and mechanisms in FSB reactions are crucial for the development of FSBs with high performances. In the present work, structural transformation, reactivities, and mechanisms in FSB reactions of BiF3 microparticles partly embedded in a gold plating film (BiF3/gold, mainly orthorhombic BiF3 (o-BiF3) containing small amounts of cubic BiF3 (c-BiF3)) were studied by in-situ Raman microscopy. Reactivities and mechanisms in defluorination were found to be different for o-BiF3 and c-BiF3. In an ionic-liquid-based electrolyte, gradual transformation of o-BiF3 into cubic c-BiF3 occurred. When the voltage of BiF3/gold (cathode) vs a Pb counter electrode (anode) was decreased from OCV (0.7 V) to 0.05 V step by step, direct defluorination of the surfaces of only o-BiF3 started from their contours at 0.45 V and then extended to their center parts and was mostly completed at 0.2 V. Then defluorination of c-BiF3 started at a voltage below 0.2 V by both direct defluorination and dissolution–deposition mechanisms. In the former mechanism, the nucleus of Bi first appeared near the edge of c-BiF3 microparticles, and the nucleus grew for defluorination to proceed over the whole surface. The rate of direct defluorination of c-BiF3 was much slower than that of o-BiF3. Defluorination of c-BiF3 by the dissolution–deposition mechanism was dominant and fast when the excitation beam was strong, probably due to a thermal effect. Such an observation is important for the development of electrodes and electrolytes with proper solubility to better utilize reactions by the two mechanisms to realize FSBs with high performance.
中文翻译:

氟化物穿梭电池反应的反应性和机理:正交和立方BiF 3单微粒之间的差异
BiF 3是氟化物穿梭电池(FSB)中正极材料的强力候选者,该电池利用金属氟化物的脱氟和金属的氟化。对于FSB,具有高能量密度的下一代电池表现出了极大的兴趣。阐明和控制FSB反应中的反应性和机理对于开发高性能FSB至关重要。另外,在本工作中,结构转换,反应性,并且在BIF的FSB反应机制3个微粒部分地嵌入在镀金膜(BIF 3 /金,主要是斜方晶系BIF 3(邻BIF 3)含有少量立方BIF的3( c-BiF 3))通过原位拉曼显微镜研究。发现对于o-BiF 3和c-BiF 3而言,脱氟的反应性和机理不同。在基于离子液体的电解质中,发生了o-BiF 3逐渐转变为立方c-BiF 3的情况。当BiF 3 /金(阴极)相对于Pb对电极(阳极)的电压逐步从OCV(0.7 V)降低到0.05 V时,仅o-BiF 3的表面从其轮廓开始直接脱氟。0.45 V,然后扩展到其中心部分,大部分在0.2 V下完成。然后c-BiF 3脱氟通过直接脱氟和溶解沉积机制在低于0.2 V的电压下开始。在前一种机制中,Bi的核首先出现在c-BiF 3微粒的边缘附近,并且该核生长以进行脱氟,从而在整个表面上进行。c-BiF 3的直接脱氟速率比o-BiF 3的慢得多。当激发光束很强时,通过溶解-沉积机理对c-BiF 3的脱氟作用占主导地位,而且速度很快,这可能是由于热效应引起的。这样的观察对于开发具有适当溶解度的电极和电解质以更好地利用两种机制的反应来实现具有高性能的FSB具有重要意义。
更新日期:2019-11-14
中文翻译:

氟化物穿梭电池反应的反应性和机理:正交和立方BiF 3单微粒之间的差异
BiF 3是氟化物穿梭电池(FSB)中正极材料的强力候选者,该电池利用金属氟化物的脱氟和金属的氟化。对于FSB,具有高能量密度的下一代电池表现出了极大的兴趣。阐明和控制FSB反应中的反应性和机理对于开发高性能FSB至关重要。另外,在本工作中,结构转换,反应性,并且在BIF的FSB反应机制3个微粒部分地嵌入在镀金膜(BIF 3 /金,主要是斜方晶系BIF 3(邻BIF 3)含有少量立方BIF的3( c-BiF 3))通过原位拉曼显微镜研究。发现对于o-BiF 3和c-BiF 3而言,脱氟的反应性和机理不同。在基于离子液体的电解质中,发生了o-BiF 3逐渐转变为立方c-BiF 3的情况。当BiF 3 /金(阴极)相对于Pb对电极(阳极)的电压逐步从OCV(0.7 V)降低到0.05 V时,仅o-BiF 3的表面从其轮廓开始直接脱氟。0.45 V,然后扩展到其中心部分,大部分在0.2 V下完成。然后c-BiF 3脱氟通过直接脱氟和溶解沉积机制在低于0.2 V的电压下开始。在前一种机制中,Bi的核首先出现在c-BiF 3微粒的边缘附近,并且该核生长以进行脱氟,从而在整个表面上进行。c-BiF 3的直接脱氟速率比o-BiF 3的慢得多。当激发光束很强时,通过溶解-沉积机理对c-BiF 3的脱氟作用占主导地位,而且速度很快,这可能是由于热效应引起的。这样的观察对于开发具有适当溶解度的电极和电解质以更好地利用两种机制的反应来实现具有高性能的FSB具有重要意义。






























 京公网安备 11010802027423号
京公网安备 11010802027423号