当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Zinc-Catalyzed Asymmetric Formal [4+3] Annulation of Isoxazoles with Enynol Ethers by 6π Electrocyclization: Stereoselective Access to 2H-Azepines.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-12-12 , DOI: 10.1002/anie.201912534 Xin-Qi Zhu 1 , Ze-Shu Wang 1 , Bo-Shang Hou 1 , Hao-Wen Zhang 1 , Chao Deng 2 , Long-Wu Ye 1, 3
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-12-12 , DOI: 10.1002/anie.201912534 Xin-Qi Zhu 1 , Ze-Shu Wang 1 , Bo-Shang Hou 1 , Hao-Wen Zhang 1 , Chao Deng 2 , Long-Wu Ye 1, 3
Affiliation
|
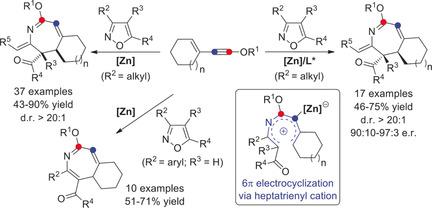
6π electrocyclization has attracted interest in organic synthesis because of its high stereospecificity and atom economy in the construction of versatile 5-7-membered cycles. However, examples of asymmetric 6π electrocyclization are quite scarce, and have to rely on the use of chiral organocatalysts, and been limited to pentadienyl-anion- and triene-type 6π electrocyclizations. Described herein is a zinc-catalyzed formal [4+3] annulation of isoxazoles with 3-en-1-ynol ethers via 6π electrocyclization, leading to the site-selective synthesis of functionalized 2H-azepines and 4H-azepines in good to excellent yields with broad substrate scope. Moreover, this strategy has also been used to produce chiral 2H-azepines with high enantioselectivities (up to 97:3 e.r.). This protocol not only is the first asymmetric heptatrienyl-cation-type 6π electrocyclization, but also is the first asymmetric reaction of isoxazoles with alkynes and the first asymmetric catalysis based on ynol ethers.
中文翻译:

锌催化的6π电环化与Enynol醚形成的异恶唑的[4 + 3]环状不对称环化:立体选择2H-Azepines。
6π电环化因其高度的立体选择性和原子经济性而在有机合成中引起了人们的兴趣,这些环在构造通用的5-7元环时非常有用。然而,不对称6π电环化的例子非常稀少,并且必须依靠手性有机催化剂的使用,并且仅限于戊二烯基阴离子和三烯型6π电环化。本文描述了经由6π电环化的异恶唑与3-en-1-ynol醚的锌催化正式[4 + 3]环化反应,从而以高至优异的收率选择性合成了功能化的2H-Azepines和4H-Azepines具有广泛的基材范围。此外,该策略也已用于生产具有高对映选择性(高达97:3 er)的手性2H--庚因。
更新日期:2019-12-13
中文翻译:

锌催化的6π电环化与Enynol醚形成的异恶唑的[4 + 3]环状不对称环化:立体选择2H-Azepines。
6π电环化因其高度的立体选择性和原子经济性而在有机合成中引起了人们的兴趣,这些环在构造通用的5-7元环时非常有用。然而,不对称6π电环化的例子非常稀少,并且必须依靠手性有机催化剂的使用,并且仅限于戊二烯基阴离子和三烯型6π电环化。本文描述了经由6π电环化的异恶唑与3-en-1-ynol醚的锌催化正式[4 + 3]环化反应,从而以高至优异的收率选择性合成了功能化的2H-Azepines和4H-Azepines具有广泛的基材范围。此外,该策略也已用于生产具有高对映选择性(高达97:3 er)的手性2H--庚因。































 京公网安备 11010802027423号
京公网安备 11010802027423号