当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ferrihydrite Growth and Transformation in the Presence of Ferrous Iron and Model Organic Ligands.
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2019-11-13 , DOI: 10.1021/acs.est.9b03952 Laurel K ThomasArrigo 1 , Ralf Kaegi 2 , Ruben Kretzschmar 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2019-11-13 , DOI: 10.1021/acs.est.9b03952 Laurel K ThomasArrigo 1 , Ralf Kaegi 2 , Ruben Kretzschmar 1
Affiliation
|
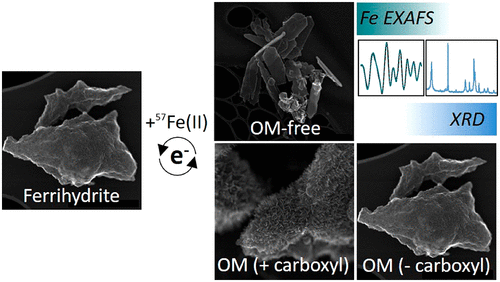
Ferrihydrite (Fh) is a poorly crystalline Fe(III)-oxyhydroxide found in abundance in soils and sediments. With a high specific surface area and sorption capacity at circumneutral pH, ferrihydrite is an important player in the biogeochemical cycling of nutrients and trace elements in redox-dynamic environments. Under reducing conditions, exposure to Fe(II) induces mineral transformations in ferrihydrite; the extent and trajectory of which may be greatly influenced by organic matter (OM). However, natural OM is heterogeneous and comprises a range of molecular weights (MWs) and varied functional group compositions. To date, the impact that the chemical composition of the associated OM has on Fe(II)-catalyzed mineral transformations is not clear. To address this knowledge gap, we coprecipitated ferrihydrite with model organic ligands selected to cover a range of MWs (25 000-50 000 vs <200 Da) as well as carboxyl content (polygalacturonic acid (PGA) > citric acid (CA) > galacturonic acid (GA)). Coprecipitates (C:Fe ≈ 0.6) were reacted with 1 mM 57Fe(II) for 1 week at pH 7, with time-resolved solid-phase analysis (via X-ray diffraction, X-ray absorption spectroscopy, and electron microscopy) revealing that all ligands inhibited Fe(II)-catalyzed ferrihydrite mineral transformations and the formation of crystalline secondary mineral phases compared to a pure ferrihydrite. For carboxyl-rich coprecipitates (Fh-PGA and Fh-CA), mineral transformations were less inhibited than in the carboxyl poor Fh-GA, and a crystalline lepidocrocite "shell" was formed surrounding the residual ferrihydrite core. However, Fe isotope analysis revealed that all coprecipitates underwent near complete atom exchange. Collectively, our results highlight that ferrihydrite is indeed an active mineral phase in redox-dynamic environments, but that its stability under reducing conditions, and thus capacity for nutrient and trace element retention, depends on the chemical characteristic of the associated OM, specifically OM-induced changes in the particle surface charge and the distribution of organic functional groups.
中文翻译:

亚铁和模型有机配体存在下水铁矿的生长和转变。
水铁矿(Fh)是一种结晶度较弱的Fe(III)-羟基氧化铁,在土壤和沉积物中含量丰富。亚铁酸盐具有高的比表面积和在环境pH值下的吸附能力,是氧化还原动态环境中养分和微量元素生物地球化学循环的重要参与者。在还原条件下,暴露于Fe(II)会引起水铁矿中的矿物转变;其范围和轨迹可能会受到有机物(OM)的极大影响。然而,天然OM是异质的,并且包括一定范围的分子量(MW)和变化的官能团组成。迄今为止,尚不清楚相关OM的化学组成对Fe(II)催化的矿物转化的影响。为了弥补这一知识鸿沟,我们将水铁矿与模型有机配体共沉淀,选择的有机配体可覆盖一定范围的MWs(25,000-50 000 vs <200 Da)以及羧基含量(聚半乳糖醛酸(PGA)>柠檬酸(CA)>半乳糖醛酸(GA))。共沉淀物(C:Fe≈0.6)与1 mM 57Fe(II)在pH 7下反应1周,并进行时间分辨固相分析(通过X射线衍射,X射线吸收光谱和电子显微镜)与纯亚铁酸盐相比,所有配体均抑制了Fe(II)催化的亚铁酸盐矿物转化和结晶次生矿物相的形成。对于富含羧基的共沉淀物(Fh-PGA和Fh-CA),与缺乏羧基的Fh-GA相比,矿物转化受到的抑制较小,并且在剩余的亚铁水合物核心周围形成了结晶的纤铁矿“壳”。然而,铁同位素分析表明,所有共沉淀物都经历了几乎完全的原子交换。总的来说,我们的研究结果表明,水铁矿确实是氧化还原动力学环境中的活性矿物相,但其在还原条件下的稳定性以及因此养分和微量元素保留的能力取决于相关OM的化学特性,特别是OM-引起颗粒表面电荷和有机官能团分布的变化。
更新日期:2019-11-13
中文翻译:

亚铁和模型有机配体存在下水铁矿的生长和转变。
水铁矿(Fh)是一种结晶度较弱的Fe(III)-羟基氧化铁,在土壤和沉积物中含量丰富。亚铁酸盐具有高的比表面积和在环境pH值下的吸附能力,是氧化还原动态环境中养分和微量元素生物地球化学循环的重要参与者。在还原条件下,暴露于Fe(II)会引起水铁矿中的矿物转变;其范围和轨迹可能会受到有机物(OM)的极大影响。然而,天然OM是异质的,并且包括一定范围的分子量(MW)和变化的官能团组成。迄今为止,尚不清楚相关OM的化学组成对Fe(II)催化的矿物转化的影响。为了弥补这一知识鸿沟,我们将水铁矿与模型有机配体共沉淀,选择的有机配体可覆盖一定范围的MWs(25,000-50 000 vs <200 Da)以及羧基含量(聚半乳糖醛酸(PGA)>柠檬酸(CA)>半乳糖醛酸(GA))。共沉淀物(C:Fe≈0.6)与1 mM 57Fe(II)在pH 7下反应1周,并进行时间分辨固相分析(通过X射线衍射,X射线吸收光谱和电子显微镜)与纯亚铁酸盐相比,所有配体均抑制了Fe(II)催化的亚铁酸盐矿物转化和结晶次生矿物相的形成。对于富含羧基的共沉淀物(Fh-PGA和Fh-CA),与缺乏羧基的Fh-GA相比,矿物转化受到的抑制较小,并且在剩余的亚铁水合物核心周围形成了结晶的纤铁矿“壳”。然而,铁同位素分析表明,所有共沉淀物都经历了几乎完全的原子交换。总的来说,我们的研究结果表明,水铁矿确实是氧化还原动力学环境中的活性矿物相,但其在还原条件下的稳定性以及因此养分和微量元素保留的能力取决于相关OM的化学特性,特别是OM-引起颗粒表面电荷和有机官能团分布的变化。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号