当前位置:
X-MOL 学术
›
Commun. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
ClpP protease activation results from the reorganization of the electrostatic interaction networks at the entrance pores.
Communications Biology ( IF 5.2 ) Pub Date : 2019-11-13 , DOI: 10.1038/s42003-019-0656-3 Mark F Mabanglo 1 , Elisa Leung 1 , Siavash Vahidi 1, 2, 3, 4 , Thiago V Seraphim 1, 5 , Bryan T Eger 1 , Steve Bryson 1, 6 , Vaibhav Bhandari 1 , Jin Lin Zhou 3 , Yu-Qian Mao 1 , Kamran Rizzolo 1 , Marim M Barghash 1 , Jordan D Goodreid 3 , Sadhna Phanse 1, 5 , Mohan Babu 5 , Leandro R S Barbosa 7 , Carlos H I Ramos 8 , Robert A Batey 3 , Lewis E Kay 1, 2, 3, 4 , Emil F Pai 1, 2, 6, 9 , Walid A Houry 1, 3
Communications Biology ( IF 5.2 ) Pub Date : 2019-11-13 , DOI: 10.1038/s42003-019-0656-3 Mark F Mabanglo 1 , Elisa Leung 1 , Siavash Vahidi 1, 2, 3, 4 , Thiago V Seraphim 1, 5 , Bryan T Eger 1 , Steve Bryson 1, 6 , Vaibhav Bhandari 1 , Jin Lin Zhou 3 , Yu-Qian Mao 1 , Kamran Rizzolo 1 , Marim M Barghash 1 , Jordan D Goodreid 3 , Sadhna Phanse 1, 5 , Mohan Babu 5 , Leandro R S Barbosa 7 , Carlos H I Ramos 8 , Robert A Batey 3 , Lewis E Kay 1, 2, 3, 4 , Emil F Pai 1, 2, 6, 9 , Walid A Houry 1, 3
Affiliation
|
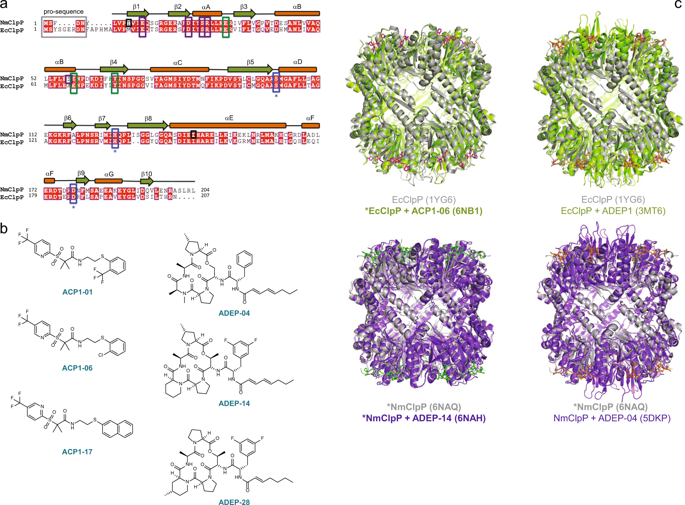
Bacterial ClpP is a highly conserved, cylindrical, self-compartmentalizing serine protease required for maintaining cellular proteostasis. Small molecule acyldepsipeptides (ADEPs) and activators of self-compartmentalized proteases 1 (ACP1s) cause dysregulation and activation of ClpP, leading to bacterial cell death, highlighting their potential use as novel antibiotics. Structural changes in Neisseria meningitidis and Escherichia coli ClpP upon binding to novel ACP1 and ADEP analogs were probed by X-ray crystallography, methyl-TROSY NMR, and small angle X-ray scattering. ACP1 and ADEP induce distinct conformational changes in the ClpP structure. However, reorganization of electrostatic interaction networks at the ClpP entrance pores is necessary and sufficient for activation. Further activation is achieved by formation of ordered N-terminal axial loops and reduction in the structural heterogeneity of the ClpP cylinder. Activating mutations recapitulate the structural effects of small molecule activator binding. Our data, together with previous findings, provide a structural basis for a unified mechanism of compound-based ClpP activation.
中文翻译:

ClpP 蛋白酶的激活是由入口孔处静电相互作用网络的重组引起的。
细菌 ClpP 是一种高度保守、圆柱形、自区室化的丝氨酸蛋白酶,是维持细胞蛋白质稳态所必需的。小分子酰基缩肽 (ADEP) 和自区室化蛋白酶 1 (ACP1) 激活剂会导致 ClpP 失调和激活,导致细菌细胞死亡,凸显了它们作为新型抗生素的潜在用途。通过 X 射线晶体学、甲基 TROSY NMR 和小角 X 射线散射探测了脑膜炎奈瑟菌和大肠杆菌 ClpP 在与新型 ACP1 和 ADEP 类似物结合后的结构变化。 ACP1 和 ADEP 诱导 ClpP 结构发生明显的构象变化。然而,ClpP 入口孔处静电相互作用网络的重组对于激活来说是必要且充分的。进一步的激活是通过形成有序的 N 端轴向环和减少 ClpP 圆柱体的结构异质性来实现的。激活突变概括了小分子激活剂结合的结构效应。我们的数据与之前的发现一起,为基于化合物的 ClpP 激活的统一机制提供了结构基础。
更新日期:2019-11-13
中文翻译:

ClpP 蛋白酶的激活是由入口孔处静电相互作用网络的重组引起的。
细菌 ClpP 是一种高度保守、圆柱形、自区室化的丝氨酸蛋白酶,是维持细胞蛋白质稳态所必需的。小分子酰基缩肽 (ADEP) 和自区室化蛋白酶 1 (ACP1) 激活剂会导致 ClpP 失调和激活,导致细菌细胞死亡,凸显了它们作为新型抗生素的潜在用途。通过 X 射线晶体学、甲基 TROSY NMR 和小角 X 射线散射探测了脑膜炎奈瑟菌和大肠杆菌 ClpP 在与新型 ACP1 和 ADEP 类似物结合后的结构变化。 ACP1 和 ADEP 诱导 ClpP 结构发生明显的构象变化。然而,ClpP 入口孔处静电相互作用网络的重组对于激活来说是必要且充分的。进一步的激活是通过形成有序的 N 端轴向环和减少 ClpP 圆柱体的结构异质性来实现的。激活突变概括了小分子激活剂结合的结构效应。我们的数据与之前的发现一起,为基于化合物的 ClpP 激活的统一机制提供了结构基础。































 京公网安备 11010802027423号
京公网安备 11010802027423号