当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of Photocrosslinking Probes Based on Huwentoxin-IV to Map the Site of Interaction on Nav1.7.
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2019-11-12 , DOI: 10.1016/j.chembiol.2019.10.011 Foteini Tzakoniati 1 , Hui Xu 2 , Tianbo Li 3 , Natalie Garcia 4 , Christine Kugel 5 , Jian Payandeh 2 , Christopher M Koth 2 , Edward W Tate 1
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2019-11-12 , DOI: 10.1016/j.chembiol.2019.10.011 Foteini Tzakoniati 1 , Hui Xu 2 , Tianbo Li 3 , Natalie Garcia 4 , Christine Kugel 5 , Jian Payandeh 2 , Christopher M Koth 2 , Edward W Tate 1
Affiliation
|
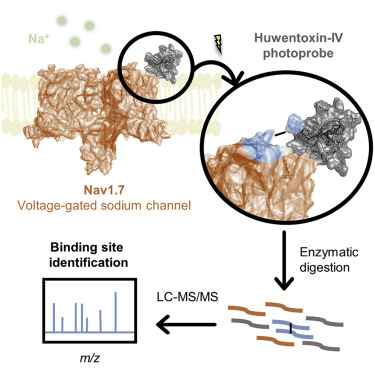
Voltage-gated sodium (Nav) channels respond to changes in the membrane potential of excitable cells through the concerted action of four voltage-sensor domains (VSDs). Subtype Nav1.7 plays an important role in the propagation of signals in pain-sensing neurons and is a target for the clinical development of novel analgesics. Certain inhibitory cystine knot (ICK) peptides produced by venomous animals potently modulate Nav1.7; however, the molecular mechanisms underlying their selective binding and activity remain elusive. This study reports on the design of a library of photoprobes based on the potent spider toxin Huwentoxin-IV and the determination of the toxin binding interface on VSD2 of Nav1.7 through a photocrosslinking and tandem mass spectrometry approach. Our Huwentoxin-IV probes selectively crosslink to extracellular loop S1-S2 and helix S3 of VSD2 in a chimeric channel system. Our results provide a strategy that will enable mapping of sites of interaction of other ICK peptides on Nav channels.
中文翻译:

基于Huwentoxin-IV的光交联探针的开发,以绘制在Nav1.7上相互作用的位点。
电压门控钠(Nav)通道通过四个电压传感器域(VSD)的协同作用来响应可兴奋细胞膜电位的变化。Nav1.7亚型在痛觉神经元的信号传播中起重要作用,并且是新型镇痛药临床开发的目标。有毒动物产生的某些抑制性胱氨酸结(ICK)肽可有效调节Nav1.7。然而,其选择性结合和活性的分子机制仍然难以捉摸。这项研究报告了基于强效蜘蛛毒素Huwentoxin-IV的光探针库的设计,以及通过光交联和串联质谱法确定Nav1.7的VSD2上的毒素结合界面的信息。我们的Huwentoxin-IV探针在嵌合通道系统中选择性交联至VSD2的细胞外环S1-S2和螺旋S3。我们的结果提供了一种策略,该策略将能够绘制Nav通道上其他ICK肽的相互作用位点。
更新日期:2019-11-13
中文翻译:

基于Huwentoxin-IV的光交联探针的开发,以绘制在Nav1.7上相互作用的位点。
电压门控钠(Nav)通道通过四个电压传感器域(VSD)的协同作用来响应可兴奋细胞膜电位的变化。Nav1.7亚型在痛觉神经元的信号传播中起重要作用,并且是新型镇痛药临床开发的目标。有毒动物产生的某些抑制性胱氨酸结(ICK)肽可有效调节Nav1.7。然而,其选择性结合和活性的分子机制仍然难以捉摸。这项研究报告了基于强效蜘蛛毒素Huwentoxin-IV的光探针库的设计,以及通过光交联和串联质谱法确定Nav1.7的VSD2上的毒素结合界面的信息。我们的Huwentoxin-IV探针在嵌合通道系统中选择性交联至VSD2的细胞外环S1-S2和螺旋S3。我们的结果提供了一种策略,该策略将能够绘制Nav通道上其他ICK肽的相互作用位点。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号