当前位置:
X-MOL 学术
›
J. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metabolic pathway analyses identify proline biosynthesis pathway as a promoter of liver tumorigenesis
Journal of Hepatology ( IF 26.8 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.jhep.2019.10.026 Zhaobing Ding 1 , Russell E Ericksen 1 , Nathalie Escande-Beillard 2 , Qian Yi Lee 1 , Abigail Loh 3 , Simon Denil 2 , Michael Steckel 4 , Andrea Haegebarth 4 , Timothy Shen Wai Ho 5 , Pierce Chow 5 , Han Chong Toh 5 , Bruno Reversade 3 , Sylvia Gruenewald 4 , Weiping Han 6
Journal of Hepatology ( IF 26.8 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.jhep.2019.10.026 Zhaobing Ding 1 , Russell E Ericksen 1 , Nathalie Escande-Beillard 2 , Qian Yi Lee 1 , Abigail Loh 3 , Simon Denil 2 , Michael Steckel 4 , Andrea Haegebarth 4 , Timothy Shen Wai Ho 5 , Pierce Chow 5 , Han Chong Toh 5 , Bruno Reversade 3 , Sylvia Gruenewald 4 , Weiping Han 6
Affiliation
|
BACKGROUND AIM
Under the regulation of various oncogenic pathways, cancer cells undergo adaptive metabolic programming to maintain specific metabolic states that support their uncontrolled proliferation. As it has been difficult to directly and effectively inhibit oncogenic signaling cascades with pharmaceutical compounds, focusing on the downstream metabolic pathways that enable indefinite growth may provide therapeutic opportunities. Thus, we sought to characterize metabolic changes in hepatocellular carcinoma (HCC) development and identify metabolic targets required for tumorigenesis. METHODS
We compared gene expression profiles of Morris Hepatoma (MH3924a) and DEN (Diethylnitrosamine)-induced HCC models to those of liver tissues from normal and rapidly regenerating liver models, and performed gain- and loss-of-function studies of the identified gene targets for their roles in cancer cell proliferation in vitro and in vivo. RESULTS
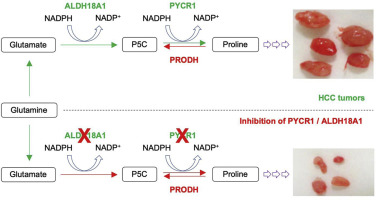
The proline biosynthetic PYCR1 (Pyrroline-5-Carboxylate Reductase 1) was identified as a top up-regulated gene in the HCC models. Knockdown (KD) of PYCR1 potently reduced cell proliferation of multiple HCC cell lines in vitro and tumor growth in vivo. Conversely, overexpression of PYCR1 enhanced the proliferation of the HCC cell lines. Importantly, PYCR1 expression was not elevated in the regenerating liver, and KD or overexpression of PYCR1 had no effect on proliferation of non-cancerous cells. Besides PYCR1, we found that additional proline biosynthetic enzymes, such as ALDH18A1, were upregulated in HCC models and also regulated HCC cell proliferation. Clinical data demonstrated that PYCR1 expression was increased in HCC, correlated with tumor grade, and was an independent predictor of clinical outcome. CONCLUSION
Enhanced expression of proline biosynthetic enzymes promotes HCC cell proliferation. Inhibition of PYCR1 or ALDH18A1 may be a novel therapeutic strategy to target HCC.
中文翻译:

代谢途径分析确定脯氨酸生物合成途径是肝脏肿瘤发生的促进者
背景目的在各种致癌途径的调节下,癌细胞经历适应性代谢编程以维持支持其不受控制的增殖的特定代谢状态。由于用药物化合物很难直接有效地抑制致癌信号级联,因此关注能够无限生长的下游代谢途径可能会提供治疗机会。因此,我们试图表征肝细胞癌(HCC)发展中的代谢变化,并确定肿瘤发生所需的代谢靶点。方法 我们将 Morris 肝癌 (MH3924a) 和 DEN(二乙基亚硝胺)诱导的 HCC 模型的基因表达谱与正常和快速再生肝模型的肝组织的基因表达谱进行比较,并对已确定的基因靶标进行功能获得和功能丧失研究因其在体外和体内癌细胞增殖中的作用。结果 脯氨酸生物合成 PYCR1(Pyrroline-5-Carboxylate Reductase 1)被确定为 HCC 模型中最上调的基因。 PYCR1 的敲低 (KD) 可有效降低多种 HCC 细胞系的体外细胞增殖和体内肿瘤生长。相反,PYCR1 的过度表达增强了 HCC 细胞系的增殖。重要的是,再生肝脏中 PYCR1 的表达并未升高,并且 KD 或 PYCR1 的过度表达对非癌细胞的增殖没有影响。除了 PYCR1 之外,我们发现其他脯氨酸生物合成酶(例如 ALDH18A1)在 HCC 模型中表达上调,并且还调节 HCC 细胞增殖。临床数据表明,HCC 中 PYCR1 表达增加,与肿瘤分级相关,并且是临床结果的独立预测因子。 结论脯氨酸生物合成酶表达增强可促进肝癌细胞增殖。抑制 PYCR1 或 ALDH18A1 可能是一种针对 HCC 的新型治疗策略。
更新日期:2020-04-01
中文翻译:

代谢途径分析确定脯氨酸生物合成途径是肝脏肿瘤发生的促进者
背景目的在各种致癌途径的调节下,癌细胞经历适应性代谢编程以维持支持其不受控制的增殖的特定代谢状态。由于用药物化合物很难直接有效地抑制致癌信号级联,因此关注能够无限生长的下游代谢途径可能会提供治疗机会。因此,我们试图表征肝细胞癌(HCC)发展中的代谢变化,并确定肿瘤发生所需的代谢靶点。方法 我们将 Morris 肝癌 (MH3924a) 和 DEN(二乙基亚硝胺)诱导的 HCC 模型的基因表达谱与正常和快速再生肝模型的肝组织的基因表达谱进行比较,并对已确定的基因靶标进行功能获得和功能丧失研究因其在体外和体内癌细胞增殖中的作用。结果 脯氨酸生物合成 PYCR1(Pyrroline-5-Carboxylate Reductase 1)被确定为 HCC 模型中最上调的基因。 PYCR1 的敲低 (KD) 可有效降低多种 HCC 细胞系的体外细胞增殖和体内肿瘤生长。相反,PYCR1 的过度表达增强了 HCC 细胞系的增殖。重要的是,再生肝脏中 PYCR1 的表达并未升高,并且 KD 或 PYCR1 的过度表达对非癌细胞的增殖没有影响。除了 PYCR1 之外,我们发现其他脯氨酸生物合成酶(例如 ALDH18A1)在 HCC 模型中表达上调,并且还调节 HCC 细胞增殖。临床数据表明,HCC 中 PYCR1 表达增加,与肿瘤分级相关,并且是临床结果的独立预测因子。 结论脯氨酸生物合成酶表达增强可促进肝癌细胞增殖。抑制 PYCR1 或 ALDH18A1 可能是一种针对 HCC 的新型治疗策略。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号