当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Overcoming Chemical Challenges in the Solid-Phase Synthesis of High-Purity GnRH Antagonist Degarelix. Part 1.
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2019-12-02 , DOI: 10.1021/acs.oprd.9b00430 Ivan Guryanov 1, 2, 3 , Andrea Orlandin 1 , Angelo Viola 1 , Barbara Biondi 2 , Denis Badocco 2 , Fernando Formaggio 2 , Antonio Ricci 1 , Walter Cabri 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2019-12-02 , DOI: 10.1021/acs.oprd.9b00430 Ivan Guryanov 1, 2, 3 , Andrea Orlandin 1 , Angelo Viola 1 , Barbara Biondi 2 , Denis Badocco 2 , Fernando Formaggio 2 , Antonio Ricci 1 , Walter Cabri 1
Affiliation
|
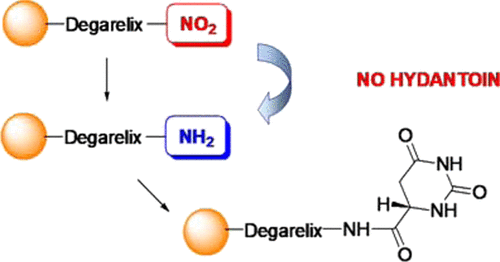
The highly potent, long-acting, gonadotropin-releasing hormone antagonist Degarelix is known to be very efficient for prostate cancer treatment. The synthesis of decapeptide Degarelix is complicated because of the presence in its sequence of several unnatural α-amino acids, which are prone to rearrangements and side reactions. In particular, the rearrangement of the dihydroorotic (Hor) moiety with following hydantoin formation in the presence of bases represents one of the major problems. In this study, we describe a novel chemical strategy to overcome this obstacle by the use of the corresponding p-nitrophenylalanine derivative, which is reduced on the solid support to p-aminophenylalanine and acylated with dihydroorotic acid at the end of the solid-phase synthesis. Thus, the contact of Hor with the bases required for Fmoc deprotection is completely avoided. This approach provides a superior purity of Degarelix when the synthesis is carried out in the industrial scale as well.
中文翻译:

克服高纯GnRH拮抗剂Degarelix固相合成中的化学挑战。第1部分。
众所周知,高效,长效促性腺激素释放激素拮抗剂Degarelix对于前列腺癌的治疗非常有效。十肽Degarelix的合成很复杂,因为其序列中存在几种非天然的α-氨基酸,这些氨基酸很容易发生重排和副反应。特别地,在碱的存在下乙内酰脲形成之后,二氢雌激素(Hor)部分的重排是主要问题之一。在这项研究中,我们描述了克服由使用相应的这个障碍的新的化学策略p -nitrophenylalanine衍生物,其上的固体支持物降低p-氨基苯丙氨酸并在固相合成结束时用二氢乳清酸酰化。因此,完全避免了Hor与Fmoc脱保护所需的碱基接触。当合成也以工业规模进行时,该方法也提供了Degarelix的优异纯度。
更新日期:2019-12-02
中文翻译:

克服高纯GnRH拮抗剂Degarelix固相合成中的化学挑战。第1部分。
众所周知,高效,长效促性腺激素释放激素拮抗剂Degarelix对于前列腺癌的治疗非常有效。十肽Degarelix的合成很复杂,因为其序列中存在几种非天然的α-氨基酸,这些氨基酸很容易发生重排和副反应。特别地,在碱的存在下乙内酰脲形成之后,二氢雌激素(Hor)部分的重排是主要问题之一。在这项研究中,我们描述了克服由使用相应的这个障碍的新的化学策略p -nitrophenylalanine衍生物,其上的固体支持物降低p-氨基苯丙氨酸并在固相合成结束时用二氢乳清酸酰化。因此,完全避免了Hor与Fmoc脱保护所需的碱基接触。当合成也以工业规模进行时,该方法也提供了Degarelix的优异纯度。































 京公网安备 11010802027423号
京公网安备 11010802027423号