当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Boosting Oxygen Evolution Reaction by Creating Both Metal Ion and Lattice-Oxygen Active Sites in a Complex Oxide.
Advanced Materials ( IF 27.4 ) Pub Date : 2019-11-12 , DOI: 10.1002/adma.201905025 Yinlong Zhu,Hassan A Tahini,Zhiwei Hu,Zhi-Gang Chen,Wei Zhou,Alexander C Komarek,Qian Lin,Hong-Ji Lin,Chien-Te Chen,Yijun Zhong,M T Fernández-Díaz,Sean C Smith,Huanting Wang,Meilin Liu,Zongping Shao
Advanced Materials ( IF 27.4 ) Pub Date : 2019-11-12 , DOI: 10.1002/adma.201905025 Yinlong Zhu,Hassan A Tahini,Zhiwei Hu,Zhi-Gang Chen,Wei Zhou,Alexander C Komarek,Qian Lin,Hong-Ji Lin,Chien-Te Chen,Yijun Zhong,M T Fernández-Díaz,Sean C Smith,Huanting Wang,Meilin Liu,Zongping Shao
|
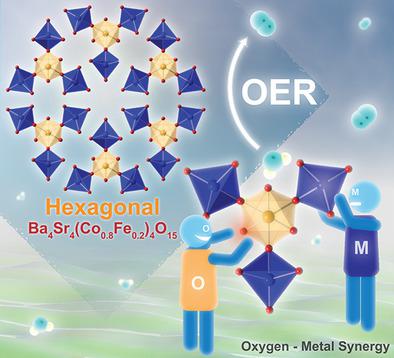
Developing efficient and low-cost electrocatalysts for the oxygen evolution reaction (OER) is of paramount importance to many chemical and energy transformation technologies. The diversity and flexibility of metal oxides offer numerous degrees of freedom for enhancing catalytic activity by tailoring their physicochemical properties, but the active site of current metal oxides for OER is still limited to either metal ions or lattice oxygen. Here, a new complex oxide with unique hexagonal structure consisting of one honeycomb-like network, Ba4 Sr4 (Co0.8 Fe0.2 )4 O15 (hex-BSCF), is reported, demonstrating ultrahigh OER activity because both the tetrahedral Co ions and the octahedral oxygen ions on the surface are active, as confirmed by combined X-ray absorption spectroscopy analysis and theoretical calculations. The bulk hex-BSCF material synthesized by the facile and scalable sol-gel method achieves 10 mA cm-2 at a low overpotential of only 340 mV (and small Tafel slope of 47 mV dec-1 ) in 0.1 m KOH, surpassing most metal oxides ever reported for OER, while maintaining excellent durability. This study opens up a new avenue to dramatically enhancing catalytic activity of metal oxides for other applications through rational design of structures with multiple active sites.
中文翻译:

通过在复合氧化物中创建金属离子和晶格氧活性位点来促进氧释放反应。
开发用于氧气释放反应(OER)的高效且低成本的电催化剂对于许多化学和能量转化技术至关重要。金属氧化物的多样性和柔韧性提供了多种自由度,可通过调整其物理化学性质来增强催化活性,但目前用于OER的金属氧化物的活性位点仍然仅限于金属离子或晶格氧。在这里,报道了一种新的具有独特六边形结构的复合氧化物,该结构由一个蜂窝状网络Ba4 Sr4(Co0.8 Fe0.2)4 O15(hex-BSCF)组成,显示出超高的OER活性,因为四面体Co离子和X射线吸收光谱分析和理论计算相结合,证实了表面上的八面体氧离子具有活性。通过简便且可扩展的溶胶-凝胶方法合成的六方体BSCF块体材料在0.1 m KOH中仅340 mV(低Tafel斜度为47 mV dec-1)的低超电势即可达到10 mA cm-2,超过了大多数金属曾有报道称氧化物可用于OER,同时保持出色的耐久性。这项研究为合理设计具有多个活性位点的结构开辟了一条新途径,可以显着提高金属氧化物的催化活性,以用于其他应用。
更新日期:2020-01-07
中文翻译:

通过在复合氧化物中创建金属离子和晶格氧活性位点来促进氧释放反应。
开发用于氧气释放反应(OER)的高效且低成本的电催化剂对于许多化学和能量转化技术至关重要。金属氧化物的多样性和柔韧性提供了多种自由度,可通过调整其物理化学性质来增强催化活性,但目前用于OER的金属氧化物的活性位点仍然仅限于金属离子或晶格氧。在这里,报道了一种新的具有独特六边形结构的复合氧化物,该结构由一个蜂窝状网络Ba4 Sr4(Co0.8 Fe0.2)4 O15(hex-BSCF)组成,显示出超高的OER活性,因为四面体Co离子和X射线吸收光谱分析和理论计算相结合,证实了表面上的八面体氧离子具有活性。通过简便且可扩展的溶胶-凝胶方法合成的六方体BSCF块体材料在0.1 m KOH中仅340 mV(低Tafel斜度为47 mV dec-1)的低超电势即可达到10 mA cm-2,超过了大多数金属曾有报道称氧化物可用于OER,同时保持出色的耐久性。这项研究为合理设计具有多个活性位点的结构开辟了一条新途径,可以显着提高金属氧化物的催化活性,以用于其他应用。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号