当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Keto–Enol Tautomerization as a First Step in Hydrogenation of Carbonyl Compounds
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-11-22 , DOI: 10.1021/acs.jpcc.9b10181 Smadar Attia 1 , Marvin C. Schmidt 1 , Carsten Schröder 1 , Jann Weber 1 , Ann-Katrin Baumann 1 , Swetlana Schauermann 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-11-22 , DOI: 10.1021/acs.jpcc.9b10181 Smadar Attia 1 , Marvin C. Schmidt 1 , Carsten Schröder 1 , Jann Weber 1 , Ann-Katrin Baumann 1 , Swetlana Schauermann 1
Affiliation
|
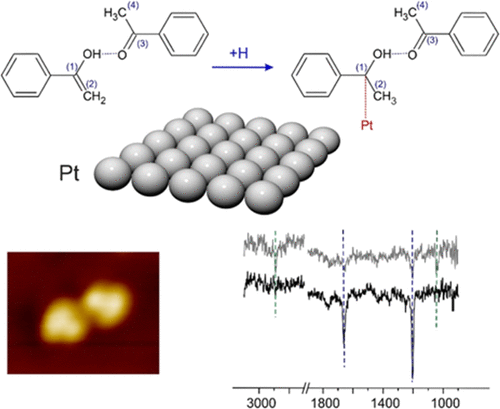
Keto–enol tautomerization of carbonyl compounds to their enol form is theoretically predicted to enable a low-barrier pathway for hydrogenation of normally very stable C═O bond. In the scope of this anticipated mechanism, the reaction can proceed via two consecutive steps, including the formation of enol followed by an H insertion into the enolic C═C bond, and exhibits a lower activation barrier than the direct H insertion into the carbonyl group. Here, we present an experimental study on atomistic level details of hydrogenation of a simple carbonyl compound acetophenone over Pt(111) providing experimental evidence that keto–enol tautomerization plays a crucial role in this reaction. By employing a combination of spectroscopic and imaging techniques, we show that acetophenone forms ketone–enol dimers, in which the normally unstable form of enol is stabilized by H-bonding with the carbonyl group of the neighboring acetophenone molecule. These ketone–enol dimers can attach an H atom to form a reaction intermediate consisting of a partly hydrogenated acetophenone species and nonhydrogenated acetophenone. Based on the spectroscopic assignment of the reaction intermediate, we conclude that H atom can be attached either to the C═C bond of the enol part, or to the strongly weakened C═O bond of the ketone part of the ketone–enol dimer. In both cases, the formation of ketone–enol dimer species was found to be a crucial step in acetophenone hydrogenation. Observed phenomena provide atomistic level insights into the mechanisms of heterogeneously catalyzed hydrogenation of simple carbonyl compounds and can be employed for purposeful modification of catalysts with functional groups capable of stabilizing the enol species.
中文翻译:

酮-烯醇互变异构是羰基化合物加氢的第一步
从理论上预测,羰基化合物的酮-烯醇互变异构成烯醇形式将为通常非常稳定的C═O键的氢化提供低阻隔途径。在这种预期机理的范围内,反应可以通过两个连续的步骤进行,包括形成烯醇,然后将H插入烯醇的C═C键中,并且比直接插入羰基中表现出更低的活化能垒。在这里,我们提供了一个简单的羰基化合物苯乙酮在Pt(111)上氢化的原子级细节的实验研究,提供了酮-烯醇互变异构在该反应中起关键作用的实验证据。通过结合使用光谱学和成像技术,我们表明苯乙酮形成了酮-烯醇二聚体,其中烯醇的通常不稳定形式是通过与相邻的苯乙酮分子的羰基进行氢键键合而稳定的。这些酮-烯醇二聚体可以连接一个H原子,形成由部分氢化的苯乙酮和未氢化的苯乙酮组成的反应中间体。基于反应中间体的光谱归属,我们得出结论,氢原子可以连接到烯醇部分的C═C键,也可以连接到酮-烯醇二聚体的酮部分的强弱C═O键。在这两种情况下,都发现酮-烯醇二聚体的形成是苯乙酮氢化的关键步骤。
更新日期:2019-11-28
中文翻译:

酮-烯醇互变异构是羰基化合物加氢的第一步
从理论上预测,羰基化合物的酮-烯醇互变异构成烯醇形式将为通常非常稳定的C═O键的氢化提供低阻隔途径。在这种预期机理的范围内,反应可以通过两个连续的步骤进行,包括形成烯醇,然后将H插入烯醇的C═C键中,并且比直接插入羰基中表现出更低的活化能垒。在这里,我们提供了一个简单的羰基化合物苯乙酮在Pt(111)上氢化的原子级细节的实验研究,提供了酮-烯醇互变异构在该反应中起关键作用的实验证据。通过结合使用光谱学和成像技术,我们表明苯乙酮形成了酮-烯醇二聚体,其中烯醇的通常不稳定形式是通过与相邻的苯乙酮分子的羰基进行氢键键合而稳定的。这些酮-烯醇二聚体可以连接一个H原子,形成由部分氢化的苯乙酮和未氢化的苯乙酮组成的反应中间体。基于反应中间体的光谱归属,我们得出结论,氢原子可以连接到烯醇部分的C═C键,也可以连接到酮-烯醇二聚体的酮部分的强弱C═O键。在这两种情况下,都发现酮-烯醇二聚体的形成是苯乙酮氢化的关键步骤。

































 京公网安备 11010802027423号
京公网安备 11010802027423号